Faculty Directory

Dr. Vivek Bagchi
Scientist-E (Associate Professor), Editorial Board Member (Energy), Scientific Reports (Nature)
Energy and Environmental Research Laboratory: We are working on the design and synthesis of new nanoscale materials for Energy Conversion/Storage, Catalysis and Environmental Remediation... Students from chemistry and physics background, can contact directly to the supervisor through email at bagchiv@inst.ac.in. For more details please visit the Personal Group page: www.vivekbagchi.in
Contact Information :
-
Email:
bagchiv@inst.ac.in -
Personal Webpage:
Personal Webpage
-
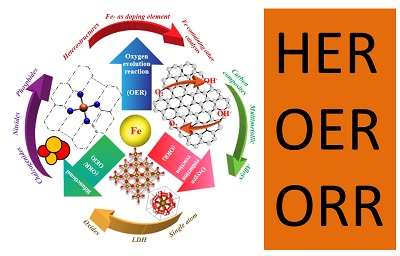
We are working on designing and synthesizing new nanoscale materials and structures with an emphasis on rational control of morphology, size, structure, composition and doping of metal carbides, nitrides, sulphides, phosphides, oxides etc on multiple scales for Energy and Environmental Applications.
Energy Conversions:
• Hydrogen Generation
• Oxygen Evolution for Air Battery applications
• Oxygen Reduction for Fuel Cell Applications
Carbon-di-Oxide Reduction
• Capture
• Conversion: Electrochemical carbon dioxide conversion
• Utilization
Energy Storage
• Supercapacitors
• Metal-ion Batteries
Environmental Remediation
• Material Development for Air/Water Purification
Catalysis
• Artificial and Biomimetic Catalysis
• C-H Bond Activation and Functionalization
-
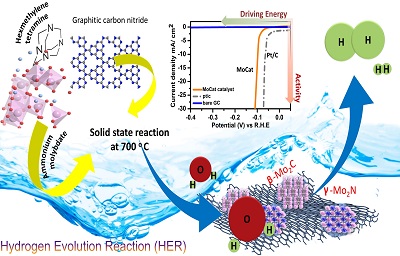
Hydrogen is one of the cleanest forms of energy which can solve several issues, including environmental pollution and depletion of fossils fuels. Hydrogen evolution reaction (HER), being a carbon-neutral process can reduce the carbon footprint in the earth’s atmosphere. Molybdenum based solids are among the most popular electrocatalysts, which have been explored extensively for HER processes as an alternative to Platinum or Platinum based materials.
-
Transition metal sulfides have been established as an excellent hydrogen evolution reaction catalyst but their oxygen evolution reaction behaviour is still less explored and needs further investigations. Oxygen evolution reaction has a huge potential for fuel cell and air battery applications. Ashish et al. have synthesized MoS2 nanolayers on N-doped carbon wrapped cobalt submicrospheres shows excellent OER catalytic activity.
-
The oxygen reduction reaction (ORR) is critically important in energy converting systems, and the advancement of a catalyst that accelerates the process is vital. Here, an ORR electrocatalyst was developed by the stoichiometric addition of Co(II) ions to a model composite (FePH) and termed as γ-CoFePH. The developed electrocatalyst shows a significant enhancement in ORR activity with improved Eonset and E1/2 (3 mA cm−2) values, where E1/2 is −0.29 V as compared to −0.60 V for FePH, drifting toward Pt/C and exhibits a limiting current density of −6.3 mA cm−2 which exceeds the commercially available Pt/C. The γ- CoFePH also displays superior resistance toward methanol poisoning and stability of over 40 000 s retaining 83.6% of the current 11 density under alkaline medium.
Current Group Members
-

RAMANDEEP SINGH
Email: ramandeep.ph24227@inst.ac.in
Reg. No.: PH24227
-

GYAN PRAKASH CHAUHAN
Email: gyan.ph24212@inst.ac.in
Reg. No.: PH24212
-

CHARU LATA GOYAL
Email: charu.ph23206@inst.ac.in
Reg. No.: PH23206
-
ALOK KUMAR
Email: alok.ph23205@inst.ac.in
Reg. No.: PH23205
-

ROHIT BISHT
Email: rohit.ph23204@inst.ac.in
Reg. No.: PH23204
-

MS. REKHA RANI
Email: rekha.ph22219@inst.ac.in
Reg. No.: PH22219
-

AASHI
Email: aashi.ph21230@inst.ac.in
Reg. No.: PH21230
-

VIKAS PUNDIR
Email: vikas.ph20235@inst.ac.in
Reg. No.: 20235_
-

KRISHANKANT
Email: krishankant.ph20236@inst.ac.in
Reg. No.: PH20236_
-

RAJDEEP KAUR
Email: rajdeep.ph21257@inst.ac.in
Reg. No.: PH21275
-

MR. DEEPAK UPRETI
Email: deepak.ph19240@inst.ac.in
Reg. No.: PH19240 (CSIR)
Alumni
-

MANU PURI
Reg. No.: INTERN
Designation: Intern Student
Jun 2025 - Aug 2025
-

KAUSTUBHI GUPTA
Reg. No.: Intern
Designation: Intern Student
Jan 2023 - Jun 2023
-

MUTHU KUMAR
Reg. No.: INST intern
Designation: Intern Student
Nov 2022 - Jan 2023
-

ARUN K
Reg. No.: INST
Designation: Intern Student
Oct 2022 - Jun 2023
-

SUBHAJIT
Reg. No.: INST Intern
Designation: Intern Student
Dec 2020 - May 2021
-

ANAND NARAYAN
Reg. No.: INT
Designation: Intern Student
May 2023 - Sep 2023
-

PAYAL THAKUR
Reg. No.: IN52
Designation: Intern Student
Jun 2023 - Apr 2024
-

ZUBAIR AHMED
Reg. No.: RP121621
Designation: PhD Scholar
Aug 2017 - Sep 2021
-

RITU RAI
Reg. No.: PH14205
Designation: PhD Scholar
Aug 2014 - Aug 2018
-

MR. RAJINDER KUMAR
Reg. No.: PH14214
Designation: PhD Scholar
Jul 2014 - Sep 2020
-

SUBHAJIT BAG
Reg. No.: INT3
Designation: Intern Student
Apr 2022 - Jun 2022
-

EKTA VERMA
Reg. No.: INT2
Designation: Intern Student
Mar 2019 - Mar 2020
-

MANALI BANSAL
Reg. No.: INST_INT_2
Designation: Intern Student
Apr 2017 - Sep 2017
-

GOURANG SAI
Reg. No.:
Designation: Intern Student
Apr 2014 - Apr 2015
-

SHREYA
Reg. No.: INT_INST_3
Designation: Intern Student
Apr 2016 - Oct 2016
-

AASHI SHARMA
Reg. No.: RP07 Aashi
Designation: Post Docs/RA
Apr 2014 - Apr 2015
-

KANIKA SONI
Reg. No.: RP07
Designation: Post Docs/RA
Apr 2016 - Apr 2017
-

MANU SHARMA
Reg. No.: PDF1619
Designation: Post Docs/RA
Apr 2015 - Apr 2016
-

KASINATH OJHA
Reg. No.: PDF1617
Designation: Post Docs/RA
Apr 2015 - Apr 2016
-

MS. SHILPA KUMARI
Reg. No.: PH18216 (INST)
Designation: PhD Scholar
Aug 2018 - Nov 2022
-

MR. ASHISH GAUR
Reg. No.: PH18212
Designation: PhD Scholar
Aug 2018 - May 2023
-

SRINIVASAN ALAGAR
Reg. No.: Postdoc_1
Designation: Post Docs/RA
Apr 2021 - Mar 2023
-

JOEL MATHEW
Reg. No.: INT5
Designation: Intern Student
May 2022 - Dec 2022
-

JATIN SHARMA
Reg. No.: INT4
Designation: Intern Student
Nov 2021 - Sep 2022
-
1.
Core-Shell Engineering of ZIF-67@N-Doped Hollow Carbon Spheres via Diffusion Strategy for Enhanced Oxygen Evolution Reaction , Deepak Upreti, Krishankant, Rekha Rani, Srinivasan Alagar, Amit Kumar Sharma, Garima Agrawal,and Vivek Bagchi , Chemistry – An Asian Journal , 2025 , e00646 , doi.org/10.1002/asia.202500646 -
2.
Interface-engineered Co4N-CeF3 heterostructure induces electronic redistribution, significantly enhances oxygen evolution at large current density , Vikas Pundir; Ashish Gaur; Rajdeep Kaur; Aashi, and Vivek Bagchi* , ACS Sustainable Chemistry & Engineering , 2025 , 13, 9 , 3491–3499 , 10.1021/acssuschemeng.4c08443 -
3.
Unveiling a Cooperative Mechanism for the Alkaline Hydrogen Evolution Reaction: Role of Built-in Electric Field , Krishankant, Rohit Bisht, Alok Kumar, Deepak Upreti, Baljeet Kaur, Rajashri R. Urkude, Chandan Bera, and Vivek Bagchi , Wiley Advanced Energy Materials , 2025 , - , 2405608 , https://doi.org/10.1002/aenm.202405608 -
4.
Topotactic transformation of zeolitic imidazolate frameworks into high-performance battery type electrodes for supercapattery application , J Sharma, S Alagar, R Kaur, A Gaur, V Pundir, D Upreti, R Rani, K Arun, V Bagchi , Dalton Transactions , 2024 , 53 , 18745-18753 , doi.org/10.1039/D4DT02507E -
5.
Electronic Redistribution Through the Interface of MnCo2O4-Ni3N Nano-Urchins Prompted Rapid In-Situ Phase Transformation for Enhanced Oxygen Evolution Reaction , Ashish Gaur, Aashi, Rajdeep Kaur, Chandan bera and Vivek Bagchi , Nanoscale , 2024 , 10663-10674 , 16 , 10.1039/D4NR00560K -
6.
Surface oxygen engineered ZnCo2O4 planar hybrid supercapacitor electrode for high energy applications , Muthukumar Ganesan a , Srinivasan Alagar a , Vivek Bagchi b , Shakkthivel Piraman a , Journal of Energy Storage , 2024 , 98, Part A , 112954 , doi.org/10.1016/j.est.2024.112954 -
7.
Electronic Reallocation in MOF-derived Co4N-Ni3N Heterostructure Renders Chlorine-Free Overall Seawater Splitting Under Large Current Density , Rajdeep Kaur, Ashish Gaur, Aashi, Vikas Pundir, Jatin Sharma, Chandan Bera, and Vivek Bagchi * , Energy and Fuels , 2024 , 11137–11147 , 38, 12, , 10.1021/acs.energyfuels.4c01543 -
8.
Unfolding the Electrocatalytic Efficiency of Ultrastable CoFeLDH Nanorods by Creating Oxygen Vacancies for OER , KrishankantAashiAyushi JainJatin SharmaRekha RaniChandan BeraVivek Bagchi* , ACS Applied Energy Materials , 2024 , 1027–1036 , 7, 3 , doi.org/10.1021/acsaem.3c02468 -
9.
Unveiling the Potential of Metal–Organic Frameworks: Nucleation-Induced Strain Activating Electrocatalytic Water Splitting , AashiAbhirup ChaudhuriKrishankantVikas PundirZubair AhmedChandan Bera*Chirodeep Bakli*Vivek Bagchi* , ACS Sustainable Chemistry & Engineering , 2024 , 12, 38 , 14276–14287 , 10.1021/acssuschemeng.4c05046 -
10.
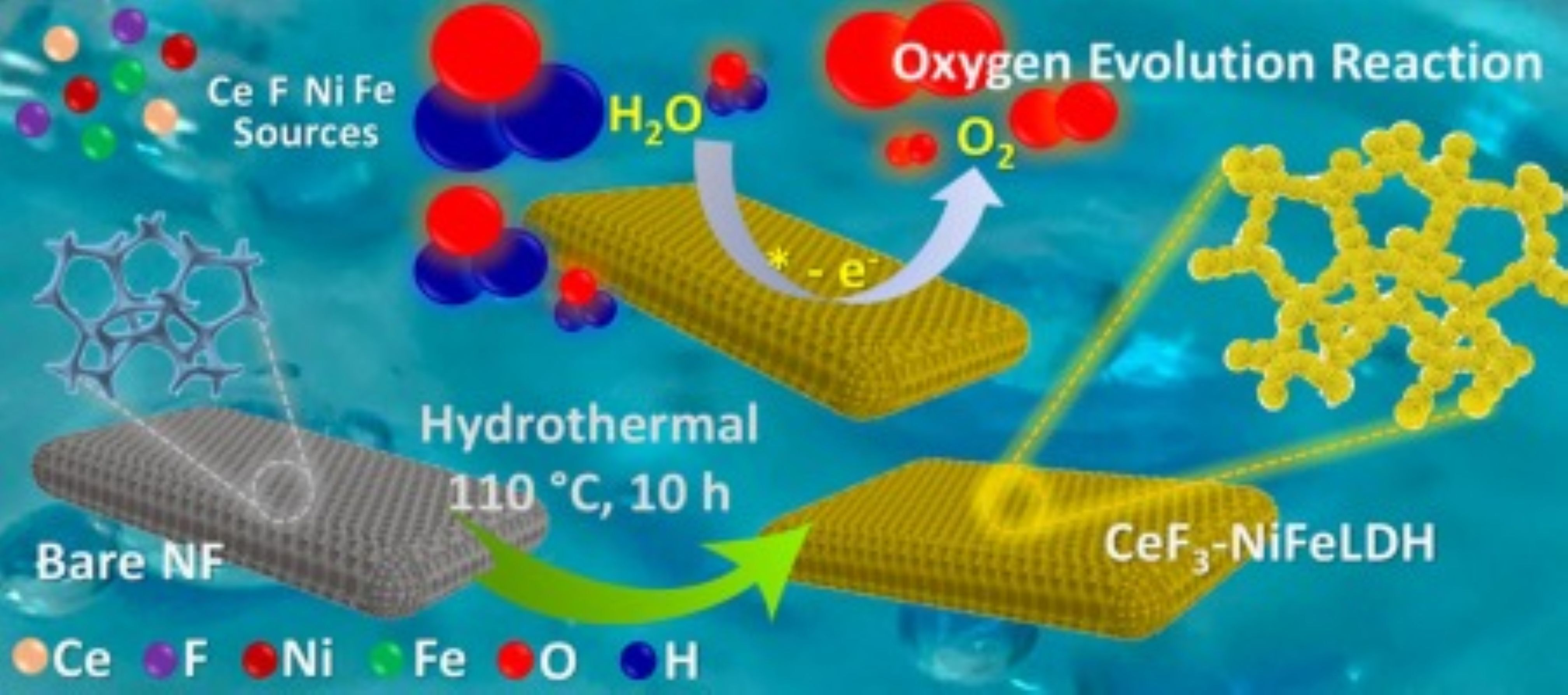
Unleashing unprecedented activation of high-valent Ni and Fe charge dynamics in CeF3-NiFe layered double hydroxide heterostructure: Demonstrating oxygen evolution reaction at an extremely high current density , Rajdeep Kaur , Ashish Gaur , Vikas Pundir , K. Arun , Vivek Bagchi , Journal of Colloid And Interface Science , 2024 , 672 , 736-743 , doi.org/10.1016/j.jcis.2024.06.034 -
11.
Laser-Induced Crafting of Modulated Structural Defects in MOF-Based Supercapacitor for Energy Storage Application , Aashi Rekha RaniSrinivasan AlagarJatin SharmaArun kVivek Bagchi* , ACS Materials Letters , 2024 , 6, 5, , 1769–1778 , 10.1021/acsmaterialslett.4c00206 -
12.
Electronic Redistribution Through the Interface: A Prodigy that Enhances Electrocatalysis , Ashish Gaur, Jatin Sharma and Vivek Bagchi* , ChemCatChem , 2023 , 16 , 10.1002/cctc.202301438 -
13.
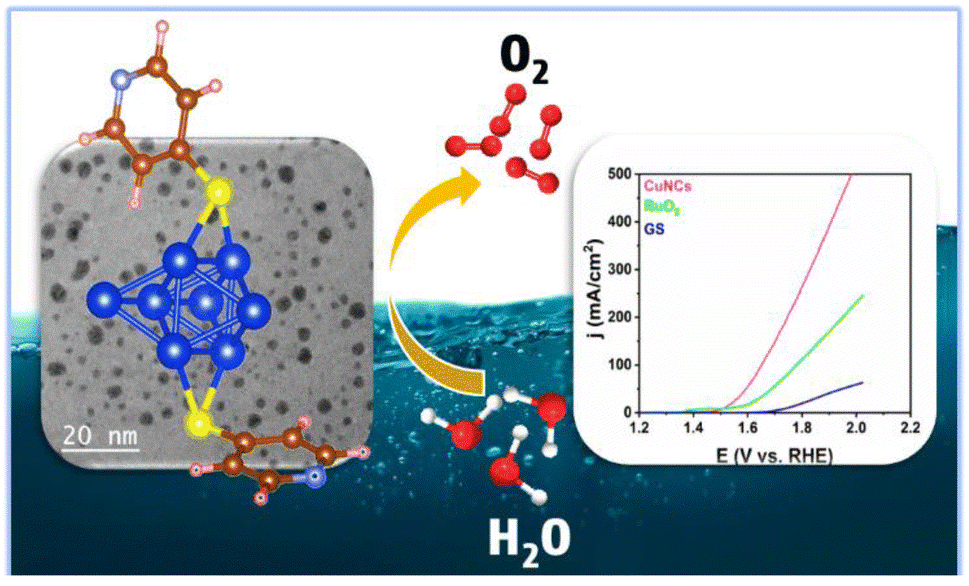
Atomically Precise Copper Nanoclusters as Potential Catalyst for Electrochemical Oxygen Evolution Reaction , Vishal Saini,† Krishankant,† Shweta Choudhary, Ashish Gaur, Swastika Banerjee,* Vivek Bagchi* and V. Venkatesh* , Journal of Materials Chemistry A , 2023 , 11 , 24754-24763 , 10.1039/D3TA04477G -
14.
Strategic Design of Li-Rich Hierarchical Li1.2Mn0.54Ni0.13Co0.13O2 Microcubes as High Capacity Cathodes for Li-ion Batteries: , Srinivasan Alagar; Muthukumar Ganesan; Chelladurai Karuppiah; Chun-Chen Yang; Vivek Bagchi; Shakkthivel Piraman , ACS Applied Energy Materials , (2023) , 6 , 622–635 , 10.1021/acsaem.2c02372 -
15.
Synergistic Modulation in a Triphasic Ni5P4-Ni2P@Ni3S2 System Manifests Remarkable Overall Water Splitting , Vikas Pundir; Ashish Gaur; Rajdeep Kaur; Jatin Sharma; Rajinder Kumar and Vivek Bagchi* , Journal of Colloids and Interface , (2023) , 651 , 579-588 , 10.1016/j.jcis.2023.07.112 -
16.
Strong Coupling effect induced surface reconstruction of CeF3-Ni3N to form CeF3-NiOOH for oxygen evolution reaction , Rajdeep Kaur,†Ashish Gaur,† Jatin Sharma, Vikas Pundir, Aashi and Vivek Bagchi* , Sustainable Energy & Fuels , (2023) , 7 , 3919-3925 , 10.1039/D3SE00679D -
17.
Rapid synthesis of CuZn-MOF via controlled electrodeposition: Manifest enhanced overall electrocatalytic water splitting , Aashi, Srinivasan Alagar, Krishankant, Ashish Gaur, Chandan Bera and Vivek Bagchi* , Sustainable Energy & Fuels , (2023) , 7 , 3692-3700 , 10.1039/D3SE00595J -
18.
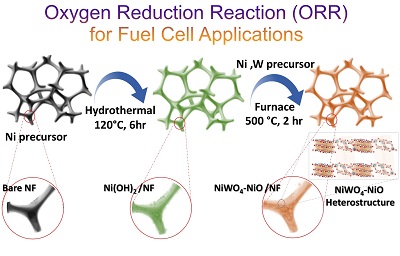
NiWO4-NiO Induces Collegial Enhancement in Hydrogen Evolution Reaction in Alkaline Medium: , Ashish Gaur; Krishankant ; Vikas Pundir; Takahiro Maruyama; Chandan Bera and Vivek Bagchi* , Journal of Colloid and Interface Science , (2023) , 641 , 82-90 , 10.1016/j.jcis.2023.02.153 -
19.
Nano-interfacial interactions in 2-D Ni3S2-Ni3N nanosheets for hydrogen evolution reaction in alkaline medium: , Vikas Pundir,† Ashish Gaur,† Rajdeep Kaur, Aashi, and Vivek Bagchi* , Energy Advances , (2023) , 2 , 321-327 , 10.1039/D2YA00296E -
20.
An Electronegativity Induced Valence States Augmentation of Ni and Co through Electronic Redistribution between Co-Ni3N/CeF3 Interfaces for Oxygen Evolution Reaction: , Ashish Gaur, Joel Mathew John, Vikas Pundir, Rajdeep Kaur, and Vivek Bagchi* , ACS Applied Energy Materials , (2023) , 6 , 1763–1770 , 10.1021/acsaem.2c03656 -
21.
3D-Hierarchical Flower like Architecture of Anion Induced Layered Double Hydroxides for Competing Anodic Reactions , Krishankant, Aashi, Baljeet Kaur, Jatin Sharma, Chandan Bera and Vivek Bagchi* , Energy Advances , (2023) , 10.1039/D3YA00188A -
22.
Curtailing the Excess eg-Orbital Filling of a Ni Atom by Enhanced Interatomic Charge Transfer within a Bimetallic 2D Metal–Organic Framework for the Oxygen Evolution Reaction , Ashish Gaur, Vikas Pundir, Rajdeep Kaur, Shambhu Nath Jha, and Vivek Bagchi* , ACS Appl. Energy Mater , (2023) , 6 , 5360–5367 , 10.1021/acsaem.3c00395 -
23.
Nano-interfaced tungsten oxide inwrought with layer double hydroxides for oxygen evolution reaction: , Krishankant ., Aashi Chauhan, Zubair Ahmed, A. Srinivasan, Ashish Gaur, Rajdeep Kaur and Vivek Bagchi* , (2022) , -: - , Sustainable Energy Fuels , https://doi.org/10.1039/D2SE00929C -
24.
High-Performance Mg-Ion Supercapacitor Designed with an N-doped Graphene wrapped CoMn2O4 and Porous Carbon Spheres: , Srinivasan Alagar, Shilpa Kumari, Deepak Upreti, Aashi, and, Vivek Bagchi* , (2022) , Energy and Fuels , https://doi.org/10.1021/acs.energyfuels.2c02760 -
25.
In-situ modulation of Al traces and interlayer spacing in Ti3C2Tx -A2 MXene: Supercapacitor with ultrahigh capacitance and energy density: , Shilpa Kumari, Srinivasan Alagar, Amit Kumar Sharma, Deepak Upreti, Aashi, Garima Agrawal, and Vivek Bagchi , (2022) , -: - , Advanced Materials and Interfaces , https://doi.org/10.1002/admi.202200919 -
26.
Valence state modulation of Mn/FePO4 nanostructures for oxygen reduction reactions: , Zubair Ahmed, Krishankant ., Parrydeep Kaur Sachdeva, Rajdeep Kaur, Shilpa Kumari, Chandan Bera and Vivek Bagchi* , (2022) , Sustainable Energy and Fuels , 10.1039/D2SE00842D -
27.
Interfacial interaction induced OER activity of MOF derived superhydrophilic Co3O4–NiO hybrid nanostructures: , Ashish Gaur, Vikas Pundir, Krishankant, Ritu Rai, Baljeet Kaur, Takahiro Maruyama, Chandan Bera and Vivek Bagchi , Dalton Transactions , (2021) , 51 , 2019-2025 , 10.1039/D1DT03810A -
28.
Current trends and perspectives on emerging Fe-derived noble metal-free oxygen electrocatalysts: , Zubair Ahmed ,Vivek Bagchi* , (2021) , -: -. , New J. Chem , https://doi.org/10.1039/D1NJ05062A -
29.
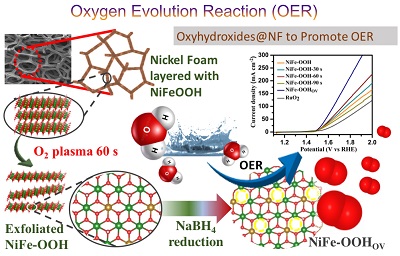
Unraveling a Graphene Exfoliation Technique Analogy in the Making of Ultrathin Nickel–Iron Oxyhydroxides@Nickel Foam to Promote the OER: , Zubair Ahmed, Krishankant, Ritu Rai, Rajinder Kumar, Takahiro Maruyama, Chandan Bera, and Vivek Bagchi , ACS Appl. Mater. Interfaces , (2021) , 13 , 55281-55291 , 10.1021/acsami.1c19536 -
30.
Intense nano-interfacial interactivity stimulates the OER in a MOF-derived superhydrophilic CuO–NiO heterostructure: , Ashish Gaur, Krishankant, Vikas Pundir, Ashwinder Singh, Takahiro Maruyama, Chandan Bera and Vivek Bagchi* , (2021) , Sustainable Energy Fuels , https://doi.org/10.1039/D1SE01235E -
31.
Micropores within N, S co-doped mesoporous 3D graphene-aerogel enhances supercapacitive performance: , Shilpa Kumari, Ekta Verma, Rajinder Kumar, Deepak Upreti, Bhanu Prakash,Takahiro Maruyama,and Vivek Bagchi* , (2021) , New Journal of Chemistry, , https://doi.org/10.1039/D1NJ00459J -
32.
Probing into the effect of heterojunctions between Cu/Mo2C/Mo2N on HER performance: , Rajinder Kumar, Zubair Ahmed, Harwinder Kaur, Chandan Bera,Vivek Bagchi* , (2020) , 10: 2213 - 2220 , Catalysis Science and Technology , https://doi.org/10.1039/C9CY02526J -
33.
Promoting electrocatalytic oxygen reduction in a model composite using selective metal ions: , Zubair Ahmed, Parrydeep K Sachdeva, Ritu Rai, Rajinder Kumar, Takahiro Maruyama,Chandan Bera ,and Vivek Bagchi* , (2020) , 3: 3645 , ACS Applied Energy Materials , https://doi.org/10.1021/acsaem.0c00129 -
34.
In-situ modulation of silica-supported MoO2/Mo2C heterojunction for enhanced hydrogen evolution reaction: , Rajinder Kumar, Zubair Ahmed,Ravi Kumar,b Shambhu Nath Jha,b Dibyendu Bhattacharyya,b Chandan Bera,and,Vivek Bagchi , (2020) , Catalysis Science and Technology , https://doi.org/10.1039/D0CY00890G -
35.
Strong Interactions between the Nanointerfaces of Silica-Supported Mo2C/MoP Heterojunction Promote Hydrogen Evolution Reaction: , Rajinder Kumar,Ashish Gaur,Takahiro Maruyama,Chandan Bera,and Vivek Bagchi* , (2020) , ACS Applied Materials & Interfaces , 10.1021/acsami.0c18196 -
36.
Ultrathin MoS2 wrapped N-doped carbon-coated cobalt nanospheres for OER application: , Ashish Gaur,Parrydeep Sachdeva,Rajinder Kumar, Takahiro Maruyama,Chandan Bera,and Vivek Bagchi* , (2020) , Sustainable Energy Fuels , https://doi.org/10.1039/D0SE01543A -
37.
Hydrated FePO4 nanoparticles supported on P-doped RGO show enhanced ORR activity compared to their dehydrated form in an alkaline medium,: , Zubair Ahmed, Ritu Rai, Rajinder Kumar,Takahiro Maruyama,and Vivek Bagchi* , (2019) , 9: 24654-24658 , RSC Advances , https://doi.org/10.1039/C9RA04070F -
38.
Uniformly Decorated Molybdenum Carbide/Nitride Nanostructures on Biomass Templates for Hydrogen Evolution Reaction Applications,: , Rajinder Kumar, Zubair Ahmed, Ritu Rai, Ashish Gaur, Shilpa Kumari, Takahiro Maruyama, Vivek Bagchi* , (2019) , 491: 4155-14161 , ACS Omega, , https://doi.org/10.1021/acsomega.9b02321 -
39.
Environmentally Benign Metal-Free Reduction of GO Using Molecular Hydrogen: A Mechanistic Insight: , Ritu Rai, Zubair Ahmed, Rajinder Kumar, Rameshwar L Kumawat, Kalyani Chordiya, Takahiro Maruyama,Md. Ehesan Ali,Vivek Bagchi* , (2018) , 3: 15112-15118 , ACS Omega , 10.1021/acsomega.8b00848 -
40.
Comparative Nitrene-Transfer Chemistry to Olefinic Substrates Mediated by a Library of Anionic Mn(II) Triphenylamido-Amine Reagents and M(II) Congeners (M = Fe, Co, Ni) Favoring Aromatic over: , Vivek Bagchi, Anshika Kalra , Purak Das, Amitava Choudhury, Zhicheng Sun , Thomas R. Cundari, and Pericles Stavropoulos , (2018) , 8: 9183–9206 , ACS Catalysis , 10.1021/acscatal.8b01941
-
Title: Embedded single/cluster atoms on the 2-D matrices for energy conversion and storage applications
PI: Dr. Vivek Bagchi
Tenure: 2023-2026
Funding Agency: DST-SERB
-
Title: Design and development of large scale prototype photochemical reactor for hydrogen generation (As Co-PI)
PI: Dr. Vivek Bagchi
Tenure: 2017-2020
Funding Agency: DST Nanomission
-
Title: Air purification device for removal of harmful gases such as ammonia, VOC’s in poultry farms (PI)
PI: Dr. Vivek Bagchi
Tenure: 2016-2019
Funding Agency: Technology Development Program
-
Title: Nano-structured Materials Synthesized from Transition Metal Carbides / Nitrides for Electrocatalytic Applications
PI: Dr. Vivek Bagchi
Tenure: 2015-2018
Funding Agency: Sanctioned by SERB, DST
-
Title: Low cost diagnostic system for public health surveillance targeting bacterial enteric pathogens (as Co-PI)
PI: Dr. Vivek Bagchi
Tenure: 2015-2018
Funding Agency: DST Nano Mission
-
Title: First-Row Transition Metals in Catalytic Atom / Group -Transfer Functionalization of Hydrocarbons
PI: Dr. Vivek Bagchi
Tenure: 2014-2017
Funding Agency: Sanctioned by SERB, DST
-
Title: Design of controlled and targeted agricultural pesticide delivery nano-carrier with copper- graphene oxide composite
PI: Dr. P.S.Vijayakumar
CO-PI: Dr. Vivek Bagchi
Tenure: 3 years
Funding Agency: DST nano mission