Faculty Directory

Dr. Shyam Lal M
Scientist-D (Assistant Professor)
Contact Information :
-
Email:
shyamlal@inst.ac.in -
Google Link:
Google Link
-
Transforming advances in nanotechnology and drug delivery into biomedical applications.
Our research thrust is to engineer delivery systems for poorly water-soluble drugs for therapeutic clinical applications, primarily against neglected infectious diseases. Also, our current team is engaged in resolving obstacles in predominant therapeutics of varicose veins, degrees of wound healing, and antibiotic resistance. We aim to develop transdermal patches which could deliver active pharmaceutical ingredients (APIs) via fabricated nanocarriers. The budding research would drive on microfluidic platforms specifically designed for controlled and sustained drug release. Conjointly, our group plans to unravel the effect of upconversion nanoparticles in light-sensitive medicines. Our delighted obsession is expanding the bounds of knowledge and possibilities in interdisciplinary areas of research and discoveries.
Current Group Members
Alumni
-

MS. AAKRITI SINGH
Reg. No.: PH17203
Designation: PhD Scholar
Aug 2017 - Feb 2023
-

SHABI PARVEZ
Reg. No.: PH15219
Designation: PhD Scholar
Jan 2016 - Mar 2022
-

MR. ABUTWAIBE KA
Reg. No.: RP852322
Designation: Project JRF/SRF
Jan 2023 - Mar 2024
-

MS. ARCHANA KAROLE
Reg. No.: PH19205
Designation: PhD Scholar
Aug 2019 - Oct 2024
-
1.
Immunotherapy and immunochemotherapy in combating visceral leishmaniasis , Yadagiri, G.; Arora, K.; Singh, A.; Mudavath, S.L.* , Front. Med. , 2023 , 10 , 1096458 , https://doi.org/10.3389/fmed.2023 -
2.
Folate receptor targeted NIR cleavable liposomal delivery system augment penetration and therapeutic efficacy in breast cancer , Dinakar, Y.H.; Karole, A.; Parvez, S.; Jain, V.; Mudavath, S.L.* , Biochimica et Biophysica Acta (BBA) - General Subjects , 2023 , 1867 , 130396 , j.bbagen.2023.130396 -
3.
Detection of Latent Fingerprints using Luminescent Gd0.95Eu0.05PO4 Nanorods , Pushpendra, Suryawanshi, I.; Kalia, R.; Kunchala, R.K.; Mudavath, S.L.; Boddu, S. N. , Journal of Rare Earth , 2022 , 40 , 572 , https://doi.org /10.1016/ j.jre. 2021. 01.015. -
4.
Downshifting and upconversion dual mode emission from lanthanide doped GdPO4 nanorods for unclonable anti-counterfeiting: , Pushpendra, I. Suryawanshi, S. Srinidhi, S. Singh, R. Kalia, R. K. Kunchala, S.L. Mudavath, B.S. Naidu* , 2021) , 26: 102144. , Mater. Today Commun. , 10.1016/j.mtcomm.2021.102144 -
5.
Carboxymethyl chitosan modified lipid nanoformulations as a highly efficacious and biocompatible oral anti-leishmanial drug carrier system: , Aakriti Singh, Ganesh Yadagiri , Manorma Negi, Anurag Kumar Kushwaha, Om Prakash Singh, Shyam Sundar, and Shyam Lal Mudavath* , (2022) , 204: 373-385 , International Journal of Biological Macromolecules , https://doi.org/10.1016/j.ijbiomac.2022.02.006 -
6.
Organ restricted delivery through stimuli responsive nanocarriers for lung cancer therapy: , Yirivinti Hayagreeva Dinakar, Archana Karole, Shabi Parvez, Vikas Jain, Shyam Lal Mudavath* , (2022) , (Just Accepted) , Life Sciences , https://doi.org/10.1016/j.lfs.2022.121133 -
7.
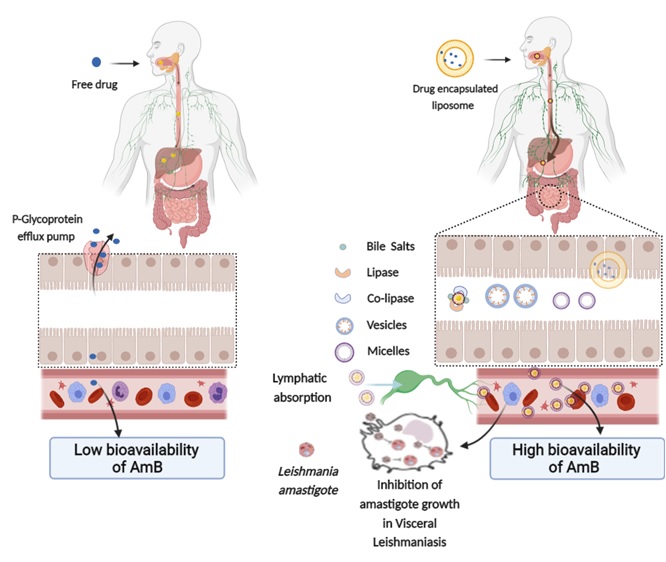
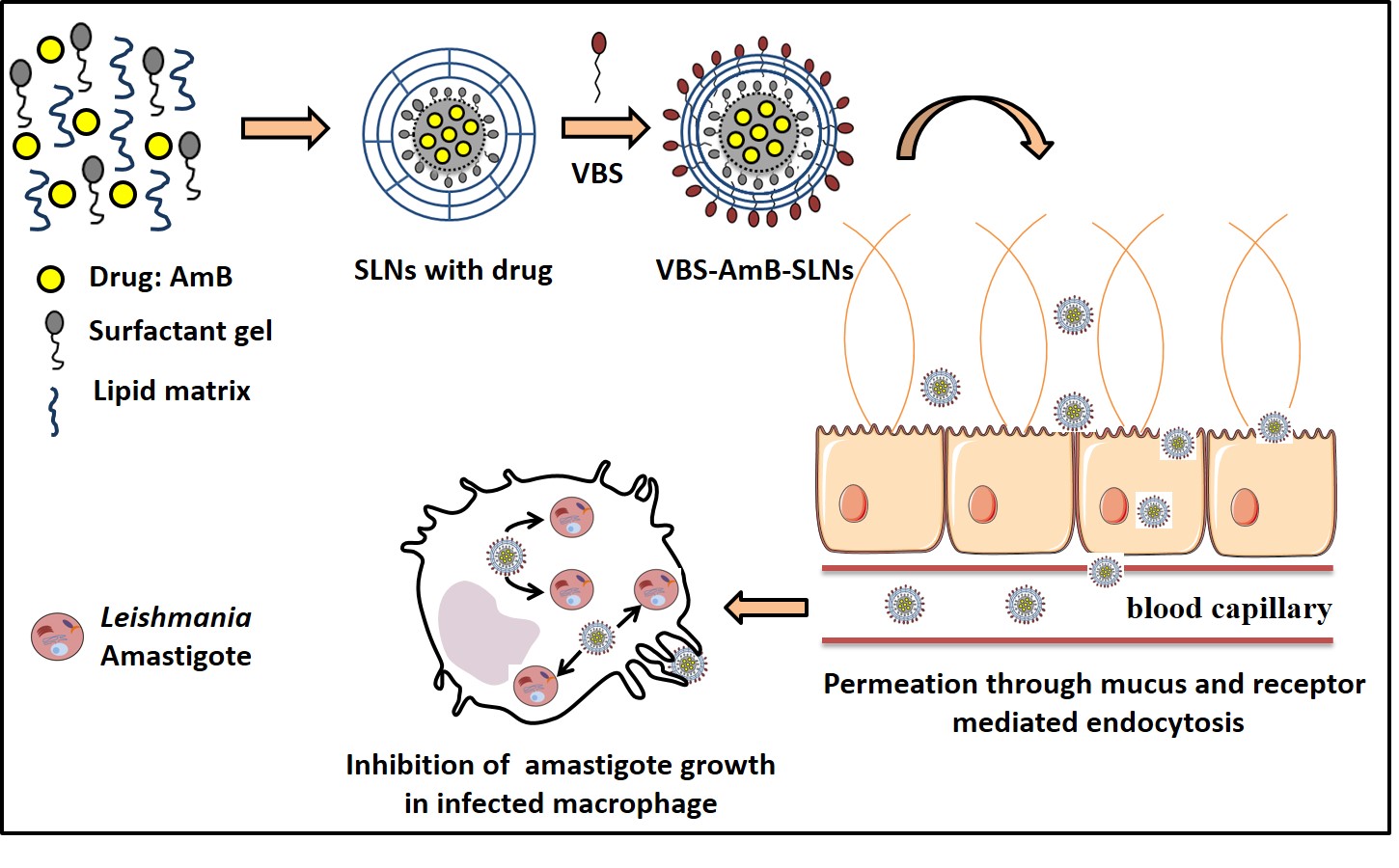
Hijacking the intrinsic Vitamin B12 pathway for oral delivery of nanoparticles ensuing an enhanced in vivo anti-leishmanial activity: , Singh, A.; Yadagiri, G.; Javaid, A.; Sharma, S.S.; Verma, A.; Singh, O.P.; Sundar, S.; Mudavath, S.L.* , (2022) , (Just Accepted): , Biomaterials Science , https://doi.org/10.1039/D2BM00979J -
8.
Transport mechanism of hydroxy-propyl-beta-cyclodextrin modified solid lipid nanoparticles across human epithelial cells for the oral absorption of antileishmanial drugs: , Shabi Parvez, Archana Karole, Shyam Lal Mudavath* , (2022) , 130157: , Biochimica et Biophysica Acta (BBA) - General Subjects , https://doi.org/10.1016/j.bbagen.2022.130157 -
9.
Fabrication, Physicochemical characterization and In vitro anticancer activity of Nerolidol encapsulated Solid Lipid Nanoparticles in Human Colorectal Cell Line: , Shabi Parvez, Archana Karole,Shyam Lal Mudavath* , (2022) , Accepted: , Colloids and Surfaces B: Biointerfaces , https://doi.org/10.1016/j.colsurfb.2022.112520 -
10.
Detection of Latent Fingerprints using Luminescent Gd0.95Eu0.05PO4 Nanorods: , Pushpendra, I. Suryawanshi, R. Kalia, R. K. Kunchala, S.L. Mudavath, B.S. Naidu* , (2022) , 40: 572-578 , J. Rare Earths , 10.1016/j.jre.2021.01.015 -
11.
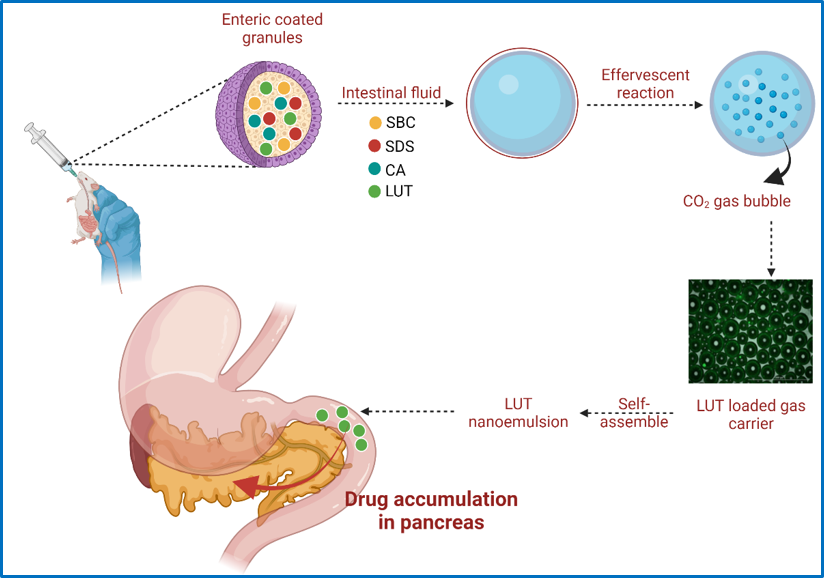
Effervescent based nano-gas carrier enhanced the bioavailability of poorly aqueous soluble drug: A comprehensive mechanistic understanding : , Archana Karole, Shabi Parvez, Richa Singh Thakur, Shyam Lal Mudavath* , (2022) , Journal of Drug Delivery Science and Technology , https://doi.org/10.1016/j.jddst.2022.103167 -
12.
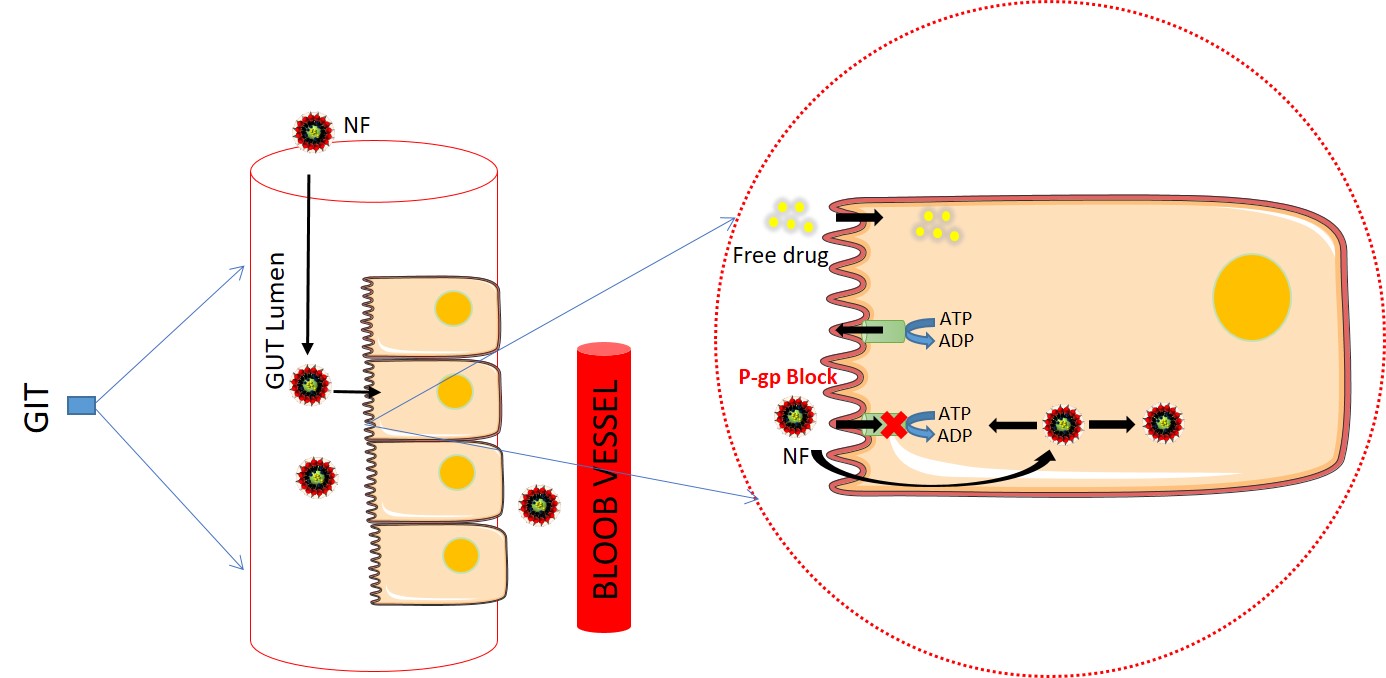
Nanoformulation mediated silencing of P-gp efflux protein for the efficient oral delivery of anti-leishmanial drugs: , Shabi Parvez, Archana Karole, Hayagreeva Dinakar Yirivinti, Shyam Lal Mudavath* , (2022) , (Just Accepted): 103959 , Journal of Drug Delivery Science and Technology , https://doi.org/10.1016/j.jddst.2022.103959 -
13.
Role of STAT3 in the initiation, progression, proliferation and metastasis of breast cancer and strategies to deliver JAK and STAT3 inhibitors: , Yirivinti Hayagreeva Dinakar, Hitesh Kumar, Shyam Lal Mudavath, Rupshee Jain Ramkishan Ajmeer Vikas Jain , (2022) , (Just Accepted): , Life Sciences , https://doi.org/10.1016/j.lfs.2022.120996 -
14.
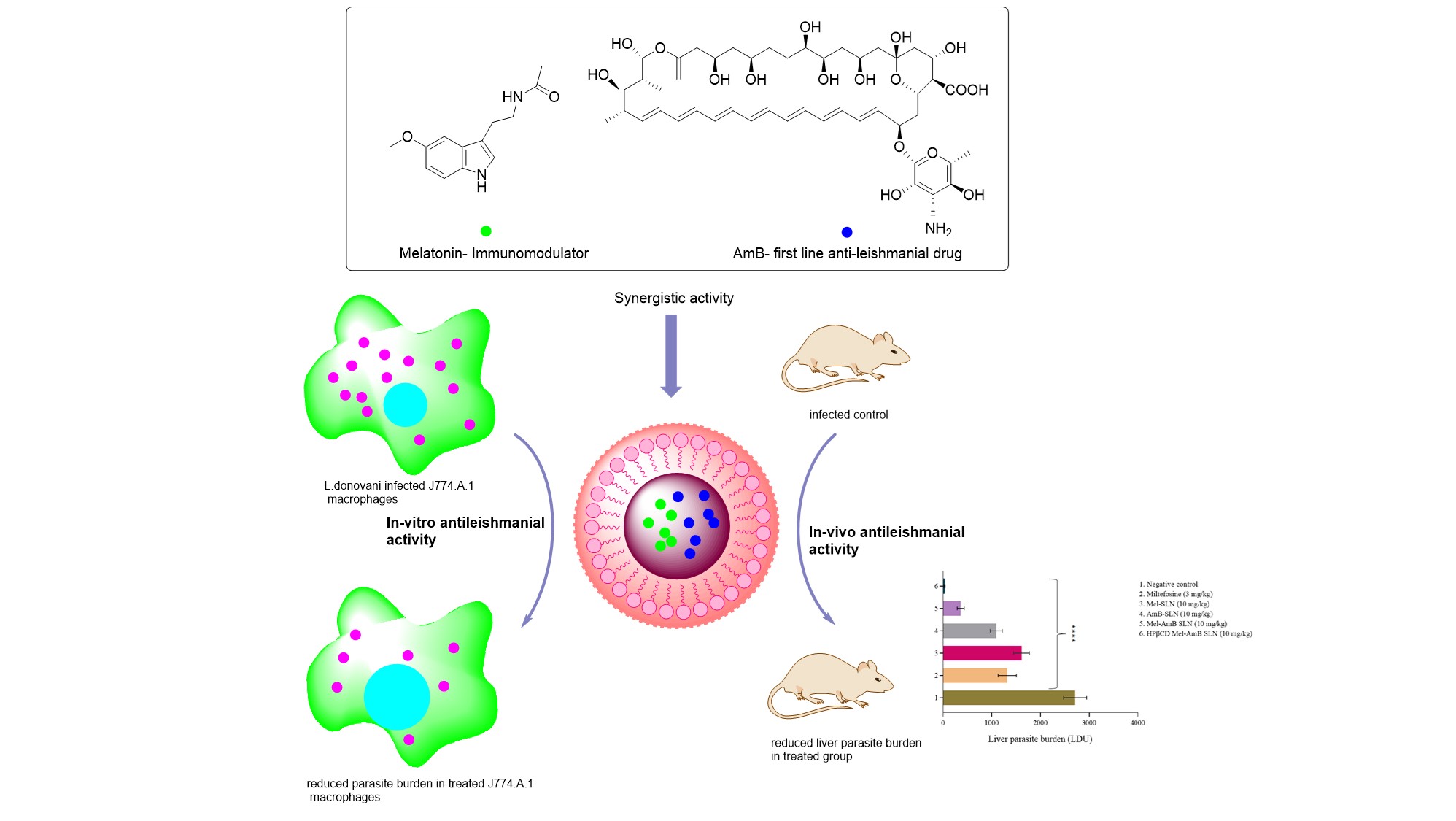
Coalition of biological agent (melatonin) with chemotherapeutic agent (amphotericin B) for combating visceral leishmaniasis via oral administration of modified solid lipid nanoparticles: , Parvez S, Yadagiri G,Arora K, Javaid A,Kushwaha A.K.,Singh O.P.,Sundar S,Mudavath S.L* , (2021) , ACS Biomaterials Science & Engineering , https://doi.org/10.1021/acsbiomaterials.1c00859 -
15.
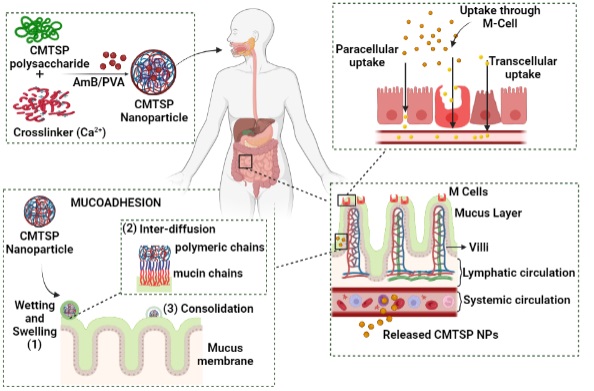
Recuperating the biopharmaceutical aspects of amphotericin B and paromomycin using a chitosan functionalized nanocarrier via oral route for enhanced anti-leishmanial activity: , Shabi Parvez, Ganesh Yadagiri, Archana Karole, Om Prakash Singh, Anurag Verma, Shyam Sundar and Shyam Lal Mudavath* , (2020) , Front. Cell. Infect. Microbiol. , 10.3389/fcimb.2020.570573 -
16.
Formulation, characterization, and in vitro anti-leishmanial evaluation of amphotericin b loaded solid lipid nanoparticles coated with vitamin B12-stearic acid conjugate: , Aakriti Singh‡, Ganesh Yadagiri‡, Shabi Parvez, Om Prakash Singh, Anurag Verma, Shyam Sundar, Shyam Lal Mudavath* , (2020) , 117: 111279 , Materials Science & Engineering C , 10.1016/j.msec.2020.111279 -
17.
Evaluation of Safety and Antileishmanial efficacy of Amine Functionalized Carbon-based Composite Nanoparticle appended with Amphotericin B: An In vitro and Preclinical Study: , Mallikarjuna Rao Gedda,Prasoon Madhukar,Alok Kumar Vishwakarm,Vimal Verma,Anurag Kumar Kushwaha,Ganesh Yadagiri,Shyam Lal Mudavath,Om Prakash Singh,Onkar Nath Srivastava,Shyam Sundar , (2020) , Front. Chem. , https://doi.org/10.3389/fchem.2020.00510 -
18.
Improvising anti-leishmanial activity of Amphotericin B and Paromomycin using co-delivery in D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) tailored Nano-lipid carrier system: , Shabi Parvez, Ganesh Yadagiri, Aakriti Singh, Archana Karole, Om Prakash Singh, Shyam Sundar,Shyam Lal Mudavath* , (2020) , 231: 104946 , Chemistry and Physics of Lipids , 10.1016/j.chemphyslip.2020.104946 -
19.
Sensible graphene oxide differentiates macrophages and Leishmania: A Bio-nano interplay in attenuating intracellular parasite: , Aakriti Singh‡, Sandeep Sharma‡, Ganesh Yadagiri, Shabi Parvez, Nikhil Koratkar, Om Prakash Singh, Shyam Sundar, Vijayakumar Shanmugam* and Shyam Lal Mudavath* , (2020) , 10: 27502–27511 , RSC Advances , DOI: 10.1039/d0ra04266h -
20.
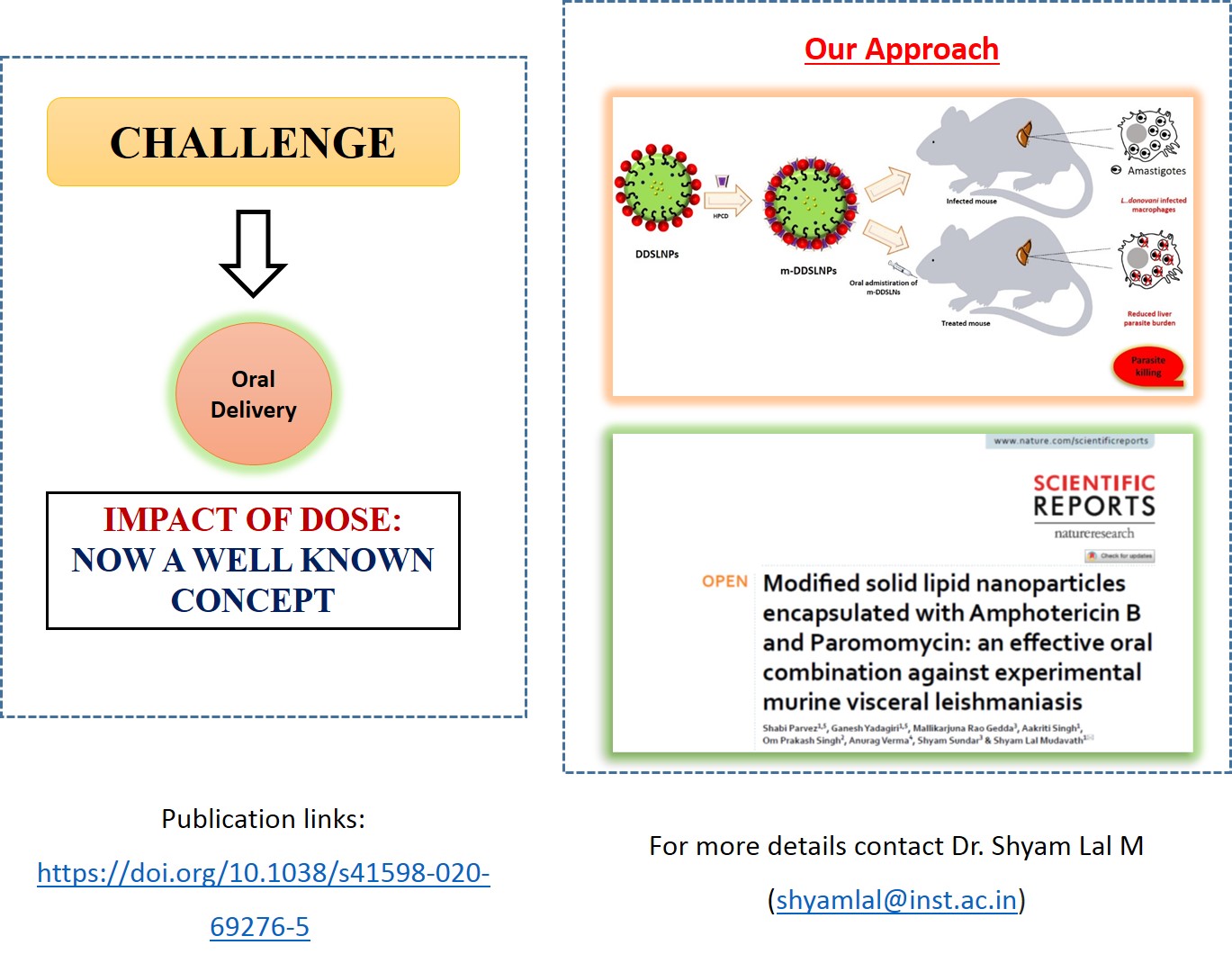
Modified solid lipid nanoparticles encapsulated with Amphotericin B and Paromomycin: An effective oral combination against experimental murine visceral leishmaniasis: , Shabi Parvez‡, Ganesh Yadagiri‡, Mallikarjuna Rao Gedda, Aakriti Singh, Om Prakash Singh, Anurag Verma, Shyam Sundar, Shyam Lal Mudavath* , (2020) , 10: 12243 , Scientific Reports , https://doi.org/10.1038/s41598-020-69276-5 -
21.
. Enkephalins as a therapeutic intervention for visceral leishmaniasis: , Ganesh Yadagiri,Shyam Lal Mudavath* , (2020) , 144: 109956 , Medical Hypotheses , doi.org/10.1016/j.mehy.2020.109956 -
22.
Nanodiagnostics in Leishmaniasis: A New Frontier for Early Elimination: , Mallikarjuna Rao Gedda, Prasoon Madhukar, Ashish Shukla, Shyam Lal Mudavath, Onkar Nath Srivastava, Om Prakash Singh and Shyam Sundar , (2020) , Accepted: 1-22 , WIREs Nanomedicine and Nanobiotechnology , 10.1002/wnan.1675 -
23.
Envisioning the innovations in nanomedicine to combat visceral leishmaniasis: for future theranostic application: , >Om Prakash Singh, Mallikarjuna Rao Gedda, Shyam Lal Mudavath, Onkar Nath Srivastava, Shyam Sundar , (2019) , 14: 1911-1927 , Nanomedicine , 10.2217/nnm-2018-0448 -
24.
Mannose-conjugated curcumin-chitosan nanoparticles: Efficacy and toxicity assessments against Leishmania donovani: , Chaubey P, Mishra B, Patel RR, Chaurasia S, Sundar S, Suvarna V, Monteiro M , (2018) , 111: 109-120 , Int J Biol Macromol , 10.1016/j.ijbiomac.2017.12.143 -
25.
Galactosylated Chitosan for Enhanced and Efficient Antimicrobial and Antileishmanial Activities: A Novel Approach: , Vishwakarma N.K.; Patel V.K.; Gundampati R.K.; Mudavath S.L.; Gupta T.; Ramesh K.; Jana K.K.; Dutta P.K.; Maiti P.; Mishra N.; Jagannadham M.V.; Sundar S.; Ray B , (2015) , 11: 11-18. DOI: NA , Asian Chitin J -
26.
In vivo assessment of antileishmanial property of 4-(4, 4, 8-Trimethyl 7-oxo-3-oxabicyclo [3.3.1] non-2-yl)-benzoic acid methyl ester, an oxabicyclo [3.3.1] nonanones, in hamster model: , Prakash Saudagar, Pipas Saha, Anil K. Saikia,Vikash Kumar Dubey,Shyam Sundar , (2014) , 11: 937-939 , Letters in Drug Design & Discovery , 10.2174/1570180811666140423203826 -
27.
Characterization and evaluation of amine modified graphene Amphotericin B for the treatment of visceral leishmaniasis: in vivo and in vitro studies: , Talat M, Rai M, Srivastava ON, Sundar S , (2014) , 8: 1235-47 , Drug Design, Development and Therapy , 10.2147/DDDT.S63994 -
28.
Comparative Evaluation of Blood and Serum Samples in Rapid Immunochromatographic Tests for Visceral Leishmaniasis: , Kumar D, Khanal B, Tiwary P, Tiwary NK, Singh R, Koirala K, Boelaert M, Rijal S, Sundar S. , (2013) , 51(12): 3955-9 , J Clin Microbiol , 10.1128/JCM.01232-13
-
1.
Current challenges and nanotechnology-based pharmaceutical approaches for the treatment and control of visceral leishmaniasis (Recent Advances in Pharmaceutical Innovation and Research) , Yadagiri, G.; Mudavath, S.L.* , 2023 , NA , NA , 9789819923014 -
2.
Promising functional Supermolecules in antiviral drugs (Pharmaceutical Applications of Supramolecules): , Arora, K.; Singh, A.; Javaid, A.; Mudavath, S.L.* , (2023) , 135-155 , Springer Cham -
3.
Vaccine human clinical trial: , Singh, B.; Shyamali, Maurya, D.K.; Kumar, R.; Chauhan, S.B.; Mudavath, S.L.; Meena, R.N.; Sundar, S.; Singh, O.P , (2022) , ISBN: 9780323859417: eBook ISBN: 9780323897860 , (Book: System Vaccinology- The History, the Translational Challenges and the Future) Elsevier -
4.
Current challenges and nanotechnology-based pharmaceutical approaches for the treatment and control of visceral leishmaniasis: , Yadagiri, G.; Mudavath, S.L.* , (2022) , (Just Accepted) , (Book: Recent Advances in Pharmaceutical Innovation and Research) Springer Nature , (Just Accepted)
-
1.
Bioimmunotherapy with Recombinant Mouse Granulocyte-Macrophage Colony-Stimulating Factor (Rm GM-CSF) and Melatonin against Experimental Visceral Leishmaniasis: , Ganesh Yadagiri, Om Prakash Singh, Shyam Sundar,Shyam Lal Mudavath* , (2020) , SSRN , https://ssrn.com/abstract=3534231</a> -
2.
An oral formulation of Amphotericin B for the treatment of visceral Leishmaniasis: f-Gr-AmB: , M Talat, M Rai, ON Srivastava, S Sundar , (2016) , 45: 3067 , International Journal Of Infectious Diseases , 10.1016/j.ijid.2016.02.790 -
3.
Amine modified graphene mediated drug delivery of Amphotericin B for the treatment of visceral leishmaniasis: , M. Rai, M. Talat, O.N. Srivastava, S. Sundar , (2014) , 21S: 1–460 , International Journal of Infectious Diseases , 10.1016/j.ijid.2014.03.753
- 1. Oxabicyclo Derivatives As Novel Antileishmanial Compounds: , Prakash Saudagar, Vikash Kumar Dubey, Anil Kumar Saikia, Pipas Saha, Shyam Lal Mudavath and Shyam Sundar , (2013) 863/KOL/2013 (Indian Patent), NA: NA
Early Career Research Award by SERB, DST, GoI.
Scientist B: Institute of Nano Science and Technology, Mohali (February 2015 to December 2018)
Scientist C: Institute of Nano Science and Technology, Mohali (January 2019 to December 2022)
Scientist D (Assistant Professor): Institute of Nano Science and Technology, Mohali (January 2023 to Present till date)
-
Title: Development of modified lipid nanovesicles based oral drug delivery system for the treatment of visceral leishmaniasis
PI: Dr. Shyam Lal M
Funding Amount: 4945968
Tenure: 3 Years
Funding Agency: SERB, DST, GoI
-
Title: Citric acid induced self-assemble bubble carrier system to enhance the oral bioavailability of poorly aqueous soluble drugs
PI: Dr. Shyam Lal M
Funding Amount: 4708840
Tenure: 3 years
Funding Agency: SERB, DST, GoI