Faculty Directory

Prof. Prakash P. Neelakandan
Scienctist 'G'
Our research group is focused on using synthetic organic chemistry to prepare new organic functional materials for applications in flexible electronic devices, sensors, and cancer therapeutics.
Contact Information :
-
Email:
ppn@inst.ac.in -
Personal Webpage:
Personal Webpage -
Google Link:
Google Link
-
Photoactive Organic Soft Materials
Our research group is focused on using synthetic organic chemistry to prepare new organic compounds and their composite materials. We have well-established laboratories and instrumentation facilities for the synthesis, characterization and studying the photochemical and photophysical properties of the newly synthesized functional materials.
The major ongoing projects include synthesis of novel luminescent organic compounds, development of flexible organic single crystals and studying plasmon-molecule coupling in organic dye loaded metal nanoparticles. These materials are then explored for applications in various fields ranging from cancer therapeutics to sensors and flexible electronic devices.
-
Highly sensitive chemosensors have been developed for biologically relevant thiols and fluoride anions.
Organic single crystals showing flexible optical waveguide and thermosalience have been developed.
Our recent results help to understand the role of chemical structure in plasmon-molecule coupling.
Dye-loaded metal nanoparticles find application as PDT and cellular imaging agents.
Current Group Members
-

ANGEL KATHPAL
Email: angel.ar240803@inst.ac.in
Reg. No.: 10CC24A67006
-

PRODIPTA SAMADDER
Email: prodipta.ph23211@inst.ac.in
Reg. No.: PH23211
-

KHALID NAIM
Email: email@inst.ac.in
Reg. No.: PH16222
-

AJAY KUMAR
Email: ajay.ph21244@inst.ac.in
Reg. No.: PH21244
-

KIRAN ARORA
Email: kiran.ph21242@inst.ac.in
Reg. No.: PH21242
-

HEMANT
Email: hemant.ar210106@inst.ac.in
Reg. No.: 10BB21J67006
-

AYUSHI NAGPAL
Email: ayushi.ph20232@inst.ac.in
Reg. No.: PH20232
-

NIDHI NAITHANI
Email: email@inst.ac.in
Reg. No.: RP7019WS1
-

PRODIPTA SAMADDER
Email: prodipta.rp942121@inst.ac.in
Reg. No.: RP942121
Alumni
-

ABHISHEK B
Reg. No.: IN-2024/02
Designation: Intern Student
Jan 2024 - Apr 2024
-

PINKI
Reg. No.: RP942322
Designation: Project JRF/SRF
Jul 2023 - Sep 2024
-

PARVATI MARANDI
Reg. No.: PH17218
Designation: PhD Scholar
Jan 2018 - Oct 2023
-

SANCHITA SHAH
Reg. No.: PH16229
Designation: PhD Scholar
Jan 2017 - Jun 2023
-

ATIKUR RAHMAN
Reg. No.: RA-01-202149
Designation: Post Docs/RA
Sep 2022 - Nov 2023
-

SUMAN R. MALLAH
Reg. No.: RP261823
Designation: Project JRF/SRF
Jan 2018 - Feb 2019
-

P. P. PRAVEEN KUMAR
Reg. No.: NPDF1709
Designation: Post Docs/RA
Oct 2017 - Jan 2020
-
1.
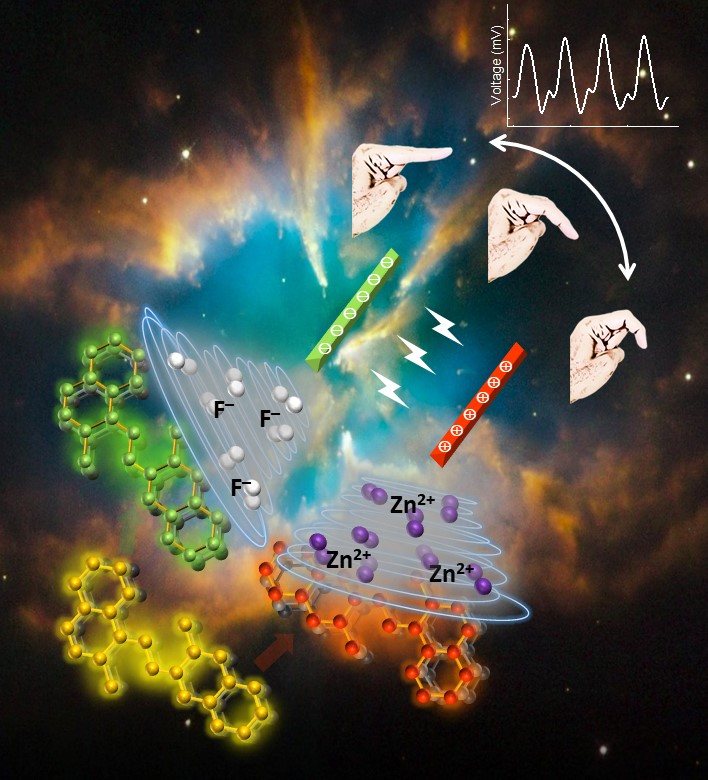
Chromophore Driven Assembly of Gold Nanobipyramids for Plasmonic and Photothermal Enhancement toward Pyroelectric Energy Harvesting , Hemant, Dalip Saini, Dipankar Mandal*, Prakash P. Neelakandan* , Nano Lett. , 2025 , - , - , 10.1021/acs.nanolett.5c03453 -
2.
Harnessing the Piezo-phototronic Effect in Flexible Chiral Organic Single Crystals for Light-Responsive Tactile Sensing , K Arora, D Saini, S Naskar, SC Sahoo, D Mandal*, PP Neelakandan* , J. Am. Chem. Soc. , 2025 , 147 , 25498-25507 , 10.1021/jacs.5c05701 -
3.
Intrinsically Pro-Apoptotic Gold Nanoclusters for Optical Tracing and Inhibition of Solid Tumors , P Sharma, H Yuan, R Verma, N Mehla, Hemant, P Sagar, C Comby-Zerbino, I Russier-Antoine, C Moulin, P-F Brevet, N Singhal, PP Neelakandan, S Vaidya, C Fu, ME Ali, R Srivastava, A Whittaker*, R Antoine*, A Shanavas* , Adv. Healthcare. Mater. , 2025 , 14 , 2405005 , 10.1002/adhm.202405005 -
4.
Sustained Acid Release from Stimuli Responsive Organic Crystals Facilitates Shape Modulation in Metal Nanoparticle Synthesis , K Naim†, P Samadder†, A Rahman, SC Sahoo, PP Neelakandan* , J. Mater. Chem. C , 2025 , 13 , 7093-7101 , 10.1039/D5TC00047E -
5.
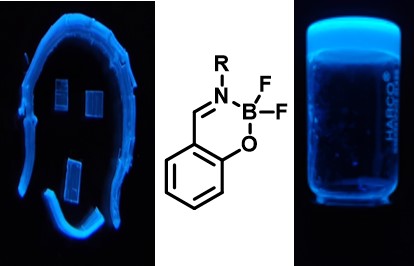
Effect of Halogenation on the Photophysics of Salicylideneimine-Boron Compound: An Unusual Behaviour with Bromination , S Bhowmik, D Mondal, K Arora, PP Neelakandan*, P Sen* , J. Photochem. Photobiol. A: Chem. , 2025 , 466 , 116361 , 10.1016/j.jphotochem.2025.116361 -
6.
An Ultrasensitive NO2 Sensor Based on Lightweight Flexible Organic Single Crystals , K Arora, N Chaudhary, SC Sahoo, K Ghosh, PP Neelakandan* , Chem. Engg. J. , 2024 , 502 , 157905 , 10.1016/j.cej.2024.157905 -
7.
Flexible Organic Molecular Single Crystal-Based Triboelectric Device as a Self-Powered Tactile Sensor , P Marandi, D Saini, K Arora, R Garg, U Sarkar, K Parida, D Mandal*, PP Neelakandan* , J. Am. Chem. Soc. , 2024 , 146 , 26178-26186 , 10.1021/jacs.4c07370 -
8.
Asymmetric Thiazolothiazole Derivative as Solvatochromic, Reversible and Self-healing Acid-Base Molecular Switch , S Shah, N Naithani, SC Sahoo, PP Neelakandan*, N Tyagi* , J. Mater. Chem. C , 2024 , 12 , 13088-13095 , 10.1039/D4TC00991F -
9.
BODIPY Directed One-Dimensional Self-assembly of Gold Nanorods , Hemant, A Rahman, P Sharma, A Shanavas, PP Neelakandan* , Nanoscale , 2024 , 16 , 12127-12133 , 10.1039/D4NR02161D -
10.
Surface Coating Induced Room-temperature Phosphorescence in Flexible Organic Single Crystals , P Samadder, K Naim, SC Sahoo, PP Neelakandan* , Chem. Sci. , 2024 , 15 , 9258-9265 , 10.1039/D4SC01708K -
11.
BODIPY-fused Uracil: Synthesis, Photophysical Properties, and Applications , A Nagpal, N Tyagi, PP Neelakandan* , Photochem. Photobiol. Sci. , 2024 , 23 , 365-376 , 10.1007/s43630-023-00524-z -
12.
Multifunctional BODIPY Embedded Non-woven Fabric for CO Release and Singlet Oxygen Generation: , S Shah, N Naithani, SC Sahoo, PP Neelakandan*, N Tyagi* , J. Photochem. Photobiol. B Biology , 2023 , 239 , 112631 , 10.1016/j.jphotobiol.2022.112631 -
13.
Plasmonically active supramolecular polymer-metal organic framework hydrogel nanocomposite for localized chemo-photothermal therapy: , N Kaur, Mimansa, P Sharma, PPP Kumar, PP Neelakandan, A Shanavas* , ACS Appl. Polym. Mater. , 2023 , 5 , 542-551 , 10.1021/acsapm.2c01646 -
14.
Selective Naked-eye Detection of Dopamine Using an Imino-Boron Molecular Capsule , PP Kumar, A Bajaj, P Samadder, ME Ali, PP Neelakandan* , New J. Chem. , 2023 , 47 , 19183-19190 , 10.1039/D3NJ02097E -
15.
Photocatalytic Degradation of Water Pollutants and Pesticides Using Plasmon-Molecule Coupled Gold-BODIPY Nanoparticles , Hemant, A Rahman, PP Neelakandan* , ChemNanoMat , 2023 , 9 , e202300236 , 10.1002/cnma.202300236 -
16.
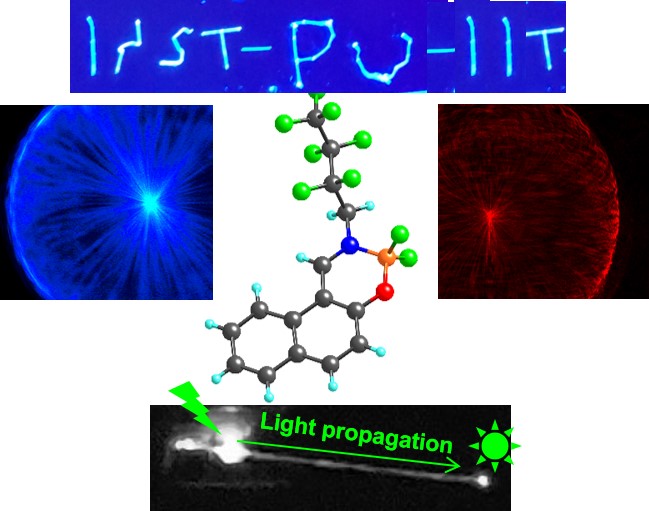
Luminescent Hexagonal Microtubes Prepared Through Water-induced Self-assembly of a Polymorphic Organoboron Compound: Formation Mechanism and Waveguide Behaviour , PA Gaikwad, P Samadder, S Som, D Chopra*, PP Neelakandan*, A Srivastava* , Nanoscale , 2023 , 15 , 14380-14387 , 10.1039/D3NR02903D -
17.
Molecular Logic Operations using Multi-stimuli Responsive, Mechanically Tunable Elastic Organic Single Crystals: , P Marandi, K Naim, K Parida, PP Neelakandan* , (2022) , 4: 2499-2505 , ACS Mater. Lett. , 10.1021/acsmaterialslett.2c00889 -
18.
Hot Electron Migration from Gold Nanoparticle to an Organic Molecule Enhances Luminescence and Photosensitization Properties of a pH Activatable Plasmon-Molecule Coupled Nanocomposite: , A Rahman, T Goswami, N Tyagi, HN Ghosh*, PP Neelakandan* , (2022) , 432: 114067 , J. Photochem. Photobiol. A: Chem , 10.1016/j.jphotochem.2022.114067 -
19.
Isomer Selective Thermosalience and Luminescence Switching in Organic Crystals: , K Naim, SC Sahoo,PP Neelakandan* , (2022) , 14: 22650-22657 , ACS Appl. Mater. Interfaces , 10.1021/acsami.2c05053 -
20.
Gold–BODIPY Nanoparticles with Luminescence and Photosensitization Properties for Photodynamic Therapy and Cell Imaging: , A Rahman, PPP Kumar, P Yadav, T Goswami, A Shanavas*, HN Ghosh*, PP Neelakandan* , (2022) , 5: 6532-6542 , ACS Appl. Nano Mater. , 10.1021/acsanm.2c00616 -
21.
Aggregation-Induced Emission, Mechanofluorochromism, and Selective Fluoride Detection by a Tripodal Salicylaldimine: , P Marandi,N Tyagi,PP Neelakandan* , (2022) , 87: e202100555 , ChemPlusChem , 10.1002/cplu.202100555 -
22.
Advances in the Supramolecular Chemistry of Tetracoordinate Boron-containing Organic Molecules into Organogels and Mesogens: , S Shah, P Marandi, PP Neelakandan* , (2021) , 9: 708854 , Front. Chem. , 10.3389/fchem.2021.708854 -
23.
Remarkable Self-assembly of Salicylideneimine-Boron Complexes into Plastic Crystals and Organogels: , K Naim, SC Sahoo, P Venugopalan, PP Neelakandan* , (2021) , 21: 3798-3806 , Cryst. Growth Des. , 10.1021/acs.cgd.1c00132 -
24.
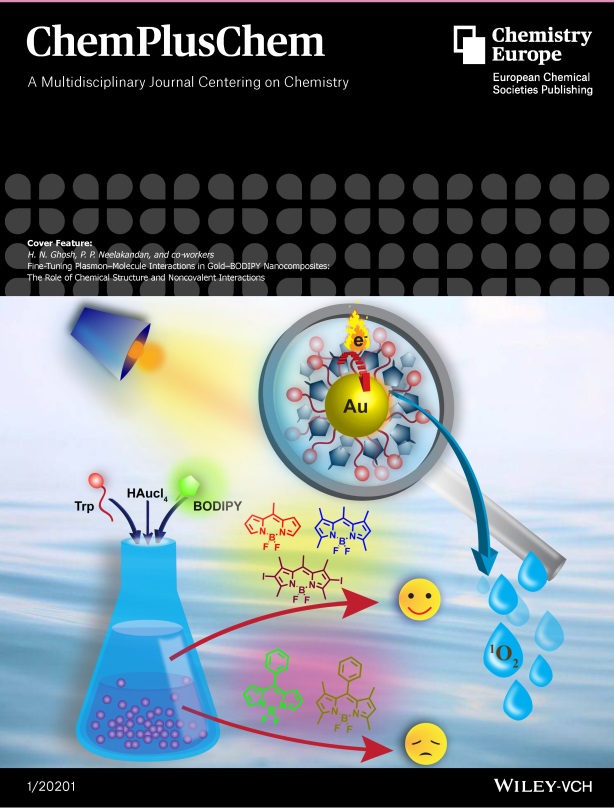
Fine‐Tuning Plasmon‐Molecule Interactions in Gold‐BODIPY Nanocomposites: The Role of Chemical Structure and Noncovalent Interactions: , PPP Kumar, A Rahman, T Goswami, HN Ghosh*, PP Neelakandan* , (2021) , 86: 87-94 , ChemPlusChem , 10.1002/cplu.202000545 -
25.
Exceptionally Plastic/Elastic Organic Crystals of a Naphthalidenimine‐Boron Complex Show Flexible Optical Waveguide Properties: , K Naim, M Singh, S Sharma, RV Nair, P Venugopalan*, SC Sahoo*, PP Neelakandan* , (2020) , 26: 11979-11984 , Chem. Eur. J. , 10.1002/chem.202002641 -
26.
Synthesis and Anti‐Proliferative Activity of a Triazole‐Fused Thymidine Analogue: , A Rahman, P Sharma, N Kaur, A Shanavas*, and PP Neelakandan* , (2020) , 5: 5473-5478 , ChemistrySelect , 10.1002/slct.202001013 -
27.
Aggregation Enhances Luminescence and Photosensitization Properties of a Hexaiodo-BODIPY: , PPP Kumar, P Yadav, A Shanavas, and PP Neelakandan* , (2020) , 4: 965-972 , Mater. Chem. Front. , 10.1039/D0QM00010H -
28.
Nanomolar Detection of Biothiols via Turn-ON Fluorescent Indicator Displacement: , PPP Kumar, N Kaur, A Shanavas, and PP Neelakandan* , (2020) , 145: 851-857 , Analyst , 10.1039/C9AN02222H -
29.
Selective Metal-ion Detection and Activatable Photosensitization Properties of a Tetraphenylethylene based Salicylideneimine: , P Marandi, PPP Kumar, P Venugopalan, and PP Neelakandan* , (2019) , 4: 5707-5713 , ChemistrySelect , 10.1002/slct.201901035 -
30.
A Three-Component Supramolecular Nanocomposite as a Heavy-Atom-Free Photosensitizer: , PPP Kumar, P Yadav, A Shanavas, S Thurakkal, J Joseph, and PP Neelakandan* , (2019) , 55: 5623-5626 , Chem. Commun. , 10.1039/C9CC02480H -
31.
Iodo-functionalized Salicylideneimine-Boron Complexes: Synthesis and Photosensitized Degradation of Organic Water Pollutants: , S Shah, A Bajaj, A Shibu, ME Ali*, and PP Neelakandan* , (2018) , 24: 18788-18794 , Chem. Eur. J. , 10.1002/chem.201804376 -
32.
Supramolecular confinement within chitosan nanocomposites enhance singlet oxygen generation: , K Naim, S T Nair, P Yadav,A Shanavas, P P Neelakandan , (2018) , 83: 418 –422. , ChemPlusChem , /10.1002/cplu.201800041 -
33.
Excitation energy delocalization and transfer to guests within M4L6 cage frameworks: , AJ Musser,† PP Neelakandan,† JM Richter, H Mori, RH Friend*, and JR Nitschke* , (2017) , 139: 12050-12059 , J. Am. Chem. Soc. , 10.1021/jacs.7b06709 -
34.
An autocatalytic system of photooxidation-driven substitution reactions on a FeII4L6 cage framework: , PP Neelakandan, A Jiménez, JD Thoburn, JR Nitschke* , (2015) , 127: 14586-14590 , Angew. Chem. Int. Ed. , 10.1002/anie.201507045 -
35.
Simultaneous binding of a cyclophane and classical intercalators to DNA: Observation of FRET-mediated white light emission: , KS Sanju, S Thurakkal, PP Neelakandan, J Joseph, D Ramaiah* , (2015) , 17: 13495-13500 , Phys. Chem. Chem. Phys. , 10.1039/C5CP00208G -
36.
Stimuli responsive metal-ligand assemblies: , AJ McConnell,† CS Wood,† PP Neelakandan,† JR Nitschke* , (2015) , 115: 7729-7793 , Chem. Rev , 10.1021/cr500632f -
37.
Fluorophore incorporation allows nanomolar guest sensing and white-light emission in M4L6 cage complexes: , PP Neelakandan, A Jiménez, JR Nitschke* , (2014) , 5: 908-915 , Chem. Sci. , 10.1039/C3SC53172D -
38.
Ground and excited state electronic spectra of perylenediimide dimers with flexible and rigid geometries in DNA conjugates: , T. A Zeidan, M. McCullagh, G. C. Schatz, J. Vura-Weis, C. H. Kim, M. R. Wasielewski, F. D. Lewis , (2014) , 5: 973-981 , Chem. Sci. , 10.1039/C3SC52908H -
39.
Interplay of monomer, intra- and intermolecular excimer fluorescence in cyclophanes and selective recognition of methanol vapours: , PC Nandajan, PP Neelakandan, D Ramaiah* , (2013) , 3: 5624-5630 , RSC Adv. , 10.1039/C3RA21678K -
40.
Facially-selective thymine-thymine photodimerization in TTT triads: , PP Neelakandan, Z Pan, M Hariharan, FD Lewis* , (2012) , 11: 889-892 , Photochem. Photobiol. Sci. , C2PP25089F
-
1.
Photoactive Finite Supramolecular Coordination Cages for Photodynamic Therapy: , N Tyagi, PP Neelakandan* , (2022) , 2023: Elsevier , Supramolecular Coordination Complexes: Design, Synthesis, and Applications , ISBN: 9780323905824
INSPIRE Faculty Award by DST, Government of India in February 2013 (not availed).
SERC Bio-inorganic Pre-doctoral Fellowship awarded by DST, Government of India in October 2006.
Postdoctoral Research Associate:University of Cambridge, UK (May 2012 to March 2015 )
Associate Professor (Scientist E):Institute of Nano Science and Technology, Mohali, India. (April 2015 to December 2020 )
Professor (Scientist F):Institute of Nano Science and Technology, Mohali, India. (January 2021 to Present till date )
Postdoctoral Fellow:Northwestern University, Evanston, IL, USA. (July 2009 to April 2012 )
-
Title: Organoboron Macrocycles as Adaptable, Photoactive Materials
PI: Dr. Prakash P. Neelakandan
Tenure: 3 years
End Date: 02-Dec-2019
Funding Agency: SERB
-
Title: Dynamic Self-assembled Nucleic Acid Analogues
PI: Dr. Prakash P. Neelakandan
CO-PI: Dr. Asifkhan Shanavas
Tenure: 3 years
End Date: 21-Feb-2021
Funding Agency: DBT
-
Title: Photoactive Organoboron Macrocycles for Sensing and Optoelectronic Applications
PI: Dr. Prakash P. Neelakandan
Tenure: 3 years
End Date: 01-Sep-2024
Funding Agency: CSIR
-
Title: Luminescent Organic Single Crystals for Flexible Display Devices
PI: Dr. Prakash P. Neelakandan
CO-PI: Dr. Kaushik Parida
Tenure: 3 years
End Date: 01-Feb-2026
Funding Agency: SERB