Faculty Directory

Dr. Rahul K. Verma: (Head of Unit)
Scientist-F (Professor)
Dr. Rahul K. Verma is a Scientist-E and Associate Professor at the Institute of Nano Science and Technology (INST), India. He served as a Visiting Scientist at Harvard Medical School (2023-24). Dr. Verma holds a PhD from Central Drug Research Institute (CDRI), Lucknow, and an MS from the National Institute of Pharmaceutical Education and Research (NIPER), Mohali. His postdoctoral research was conducted at the University of Oklahoma, USA, and he was a Visiting Researcher at Bradford University, United Kingdom, with sponsorship from the Royal Society. Dr. Verma has authored over 100 peer-reviewed articles, with an H-index of 32. He has received awards such as the CDRI Incentive Award and the BRG-France Travel Award twice. His research focuses on developing pharmaceuticals for Tuberculosis, lung cancer, Frostbite, Rheumatoid arthritis, including designing new therapeutic peptides, repurposing drugs, and creating novel formulations.
Contact Information :
-
Email:
rahulverma@inst.ac.in -
Google Link:
Google Link
-
Our research is centered on the development of safe, efficient, and clinically viable pharmaceutical biomaterials and nanoparticulate delivery systems that enable targeted, controlled release and depot effect of therapeutic agents at their site of action. We focus on the design, development, and evaluation of polymeric particulate drug delivery systems, including microparticles, nanoparticles, and porous nanoparticle aggregates (PNAP), tailored for pulmonary, nasal, transdermal, intraarticular, and transdermal administration routes. Additionally, we are involved in designing pharmaceutical formulations for veterinary purposes.
A significant aspect of our research involves the delivery of therapeutic agents for the treatment of lung and systemic diseases via dry powder inhalation (DPIs). Our work encompasses both in vitro and in vivo evaluations of novel drug delivery platforms, including preclinical drug testing in cell lines and animal models. We are particularly focused on addressing critical health challenges such as tuberculosis, lung cancer, frostbite, rheumatoid arthritis, and high altitude pulmonary edema (HAPE). Our research extends to applications in both human and veterinary medicine, with the goal of advancing therapeutic outcomes through innovative delivery systems.
-
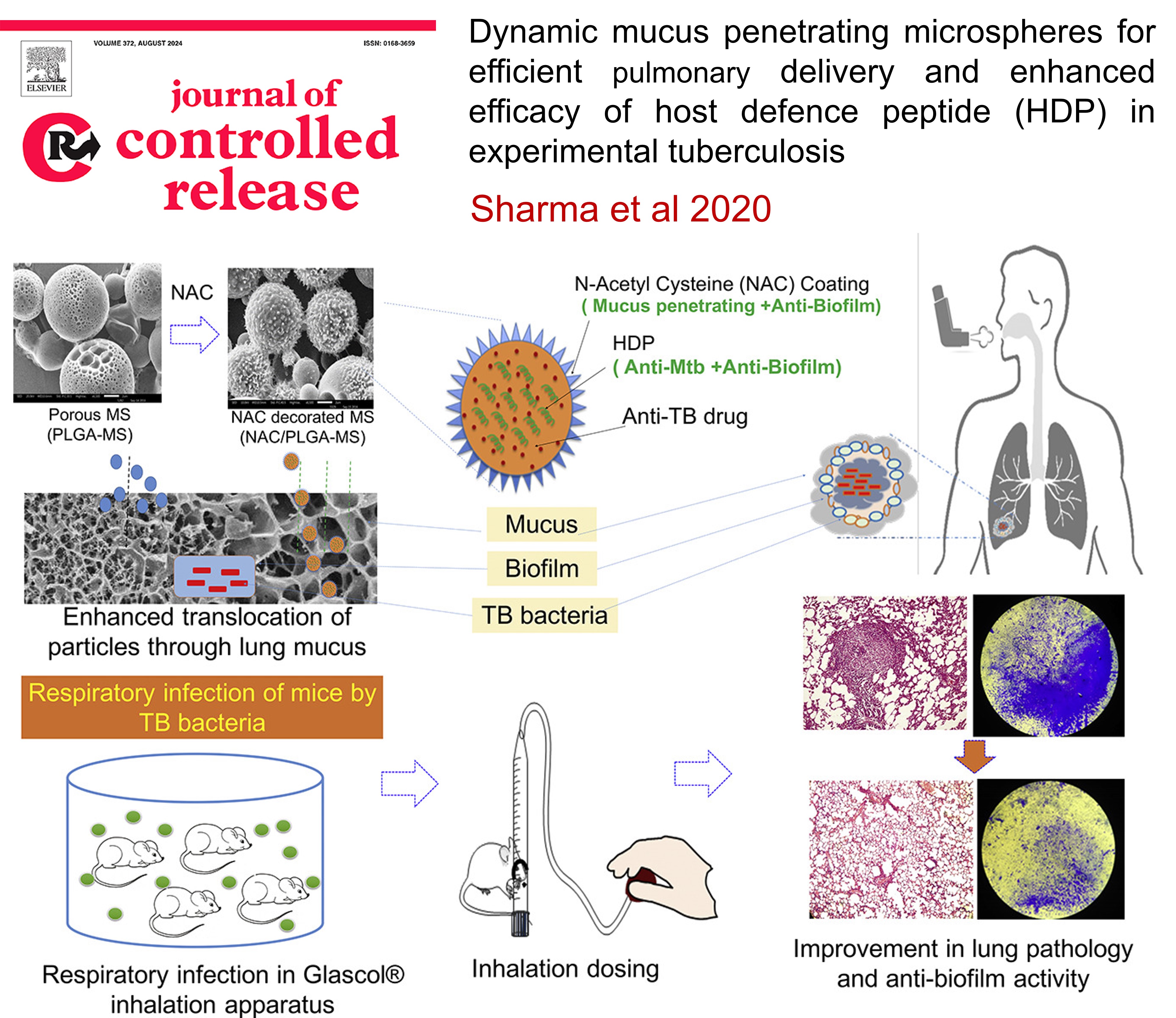
PULMONARY TUBERCULOSIS: Dynamic mucus penetrating microspheres for efficient pulmonary delivery and enhanced efficacy of host defence peptide (HDP) in experimental tuberculosis
Pulmonary drug delivery for tuberculosis (TB) can enhance treatment by targeting high drug concentrations directly at the infection site while minimizing systemic toxicity. Conventional inhalable microparticles often face challenges like mucus entrapment and quick clearance, reducing their effectiveness. This study developed mucus-penetrating microparticles (NAC/PLGA-MPP) that enhance drug delivery and disrupt bacterial biofilms. In a TB mouse model, these microparticles significantly reduced bacterial load and lung inflammation, showing promise as an adjunct therapy to existing TB treatments like DOTS
-
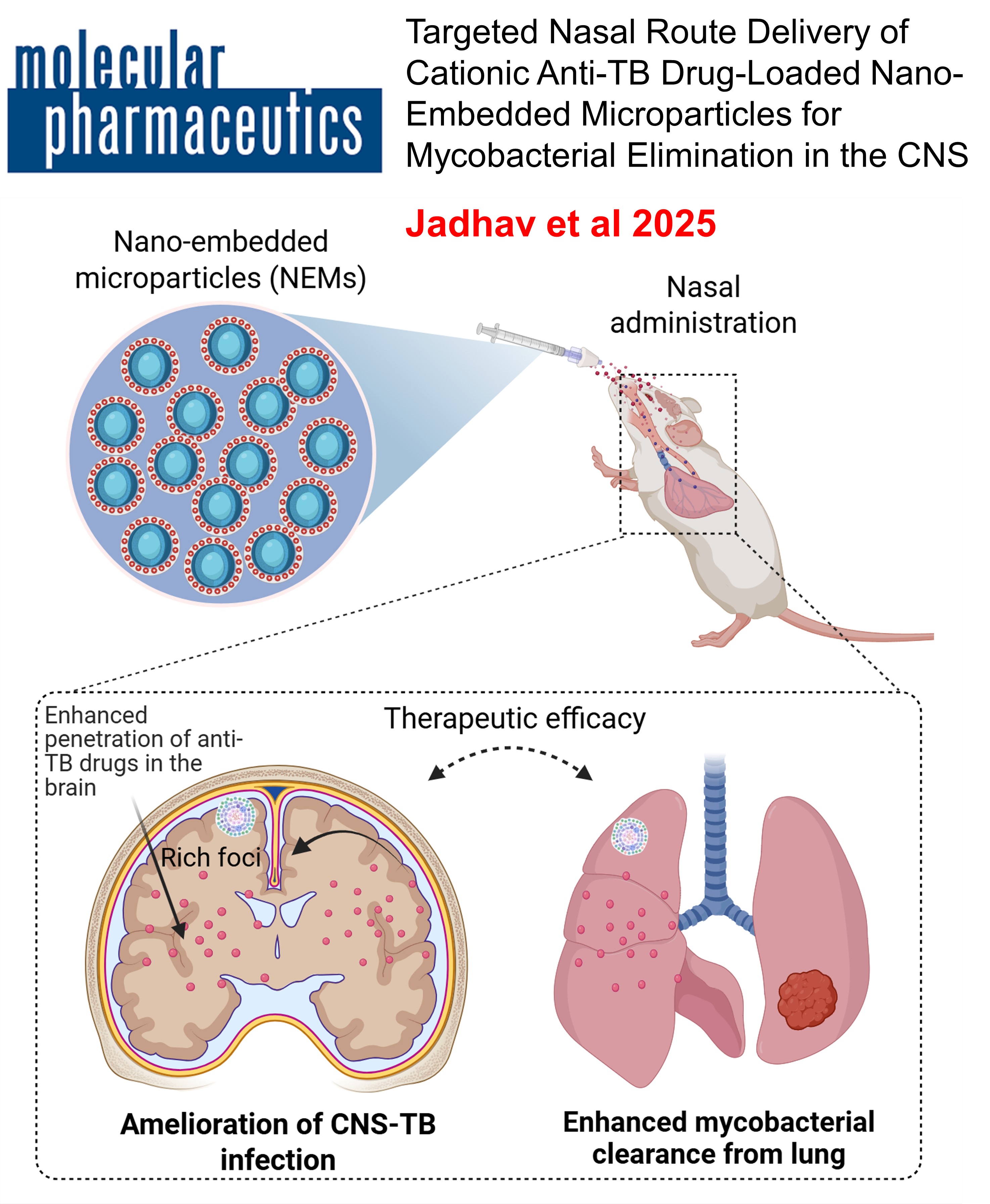
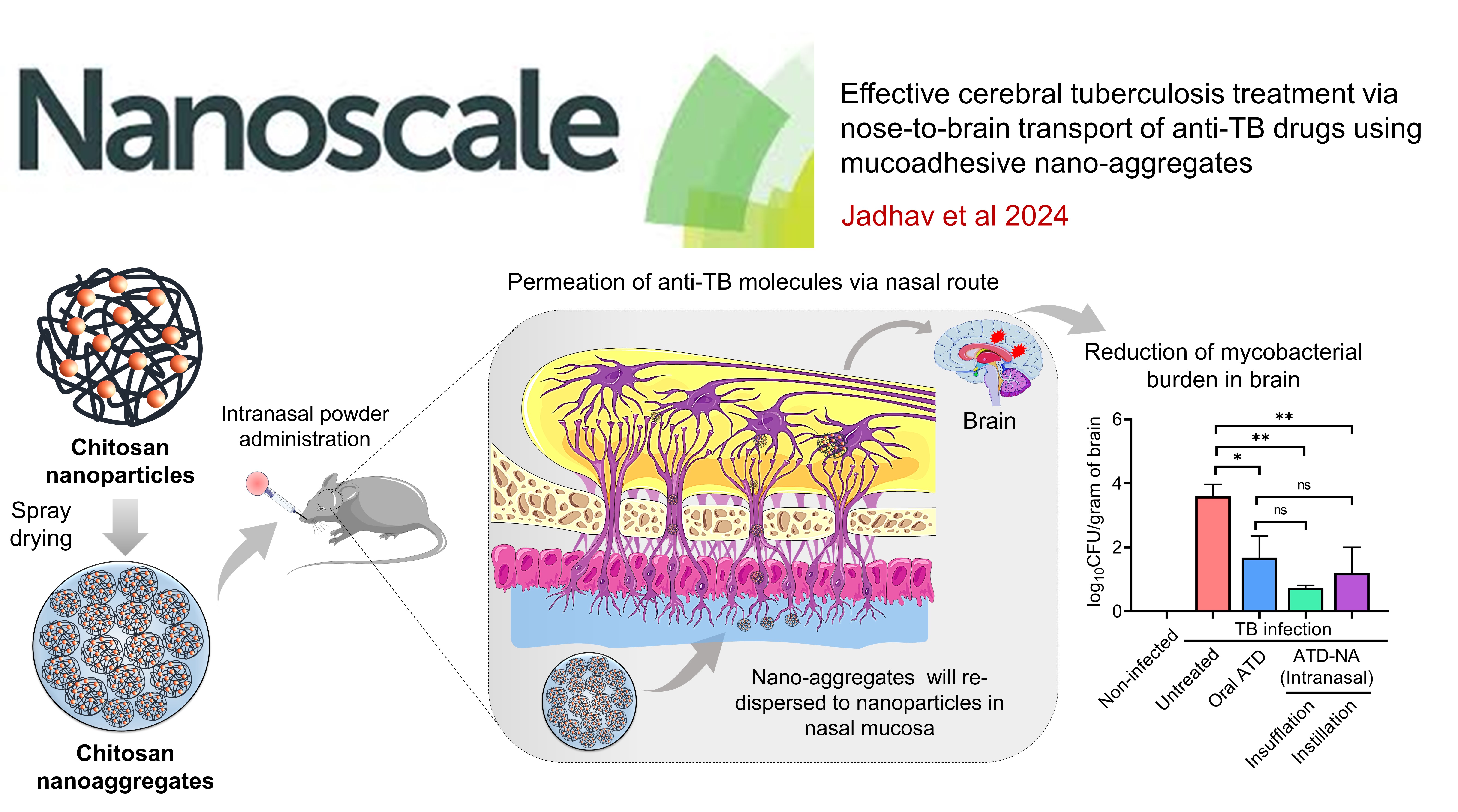
CEREBRAL TUBERCULOSIS: Effective cerebral tuberculosis treatment via nose-to-brain transport of anti-TB drugs using mucoadhesive nano-aggregates
This study developed chitosan nanoparticles encapsulating anti-TB drugs (isoniazid and rifampicin) for nasal delivery to treat central nervous system tuberculosis (CNS-TB). The nanoparticles were processed into micro-sized chitosan nano-aggregates (NA) via spray drying. The intranasal administration of these nano-aggregates to TB-infected mice showed significantly higher drug permeation and mucoadhesion compared to free drugs. Over four weeks, the treatment led to a significant reduction in mycobacterial load (~2.86 Log10 CFU) in the mice. This preclinical study demonstrates the high therapeutic potential of nasal chitosan nano-aggregates in treating CNS-TB, offering a promising approach to bypass the blood-brain barrier and target brain infections effectively.
-
COLD INJURY/ FROST BITE: Heparin-Encapsulated Metered-Dose Topical “Nano-Spray Gel” Liposomal Formulation Ensures Rapid On-Site Management of Frostbite Injury by Inflammatory Cytokines Scavenging
The time between frostbite injury and treatment initiation in remote areas is crucial for an effective response. Frostbite is challenging to treat due to poor blood flow and requires immediate intervention. This study developed a topical nano-spray gel (NSG) combining liposomal heparin and ibuprofen (HLp-Ibu-NSG) for rapid frostbite relief. Heparin promotes wound healing and normalizes blood circulation, while ibuprofen reduces inflammation. In a rat model, the NSG significantly reduced wound area (up to 96%) and improved healing within 14 days without causing edema or erythema. The formulation modulates inflammatory cytokines, suggesting potential for on-site treatment in frostbite cases.
-
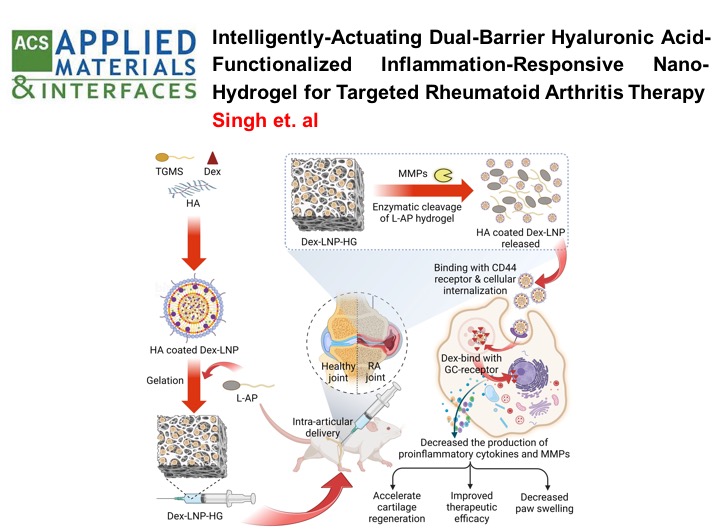
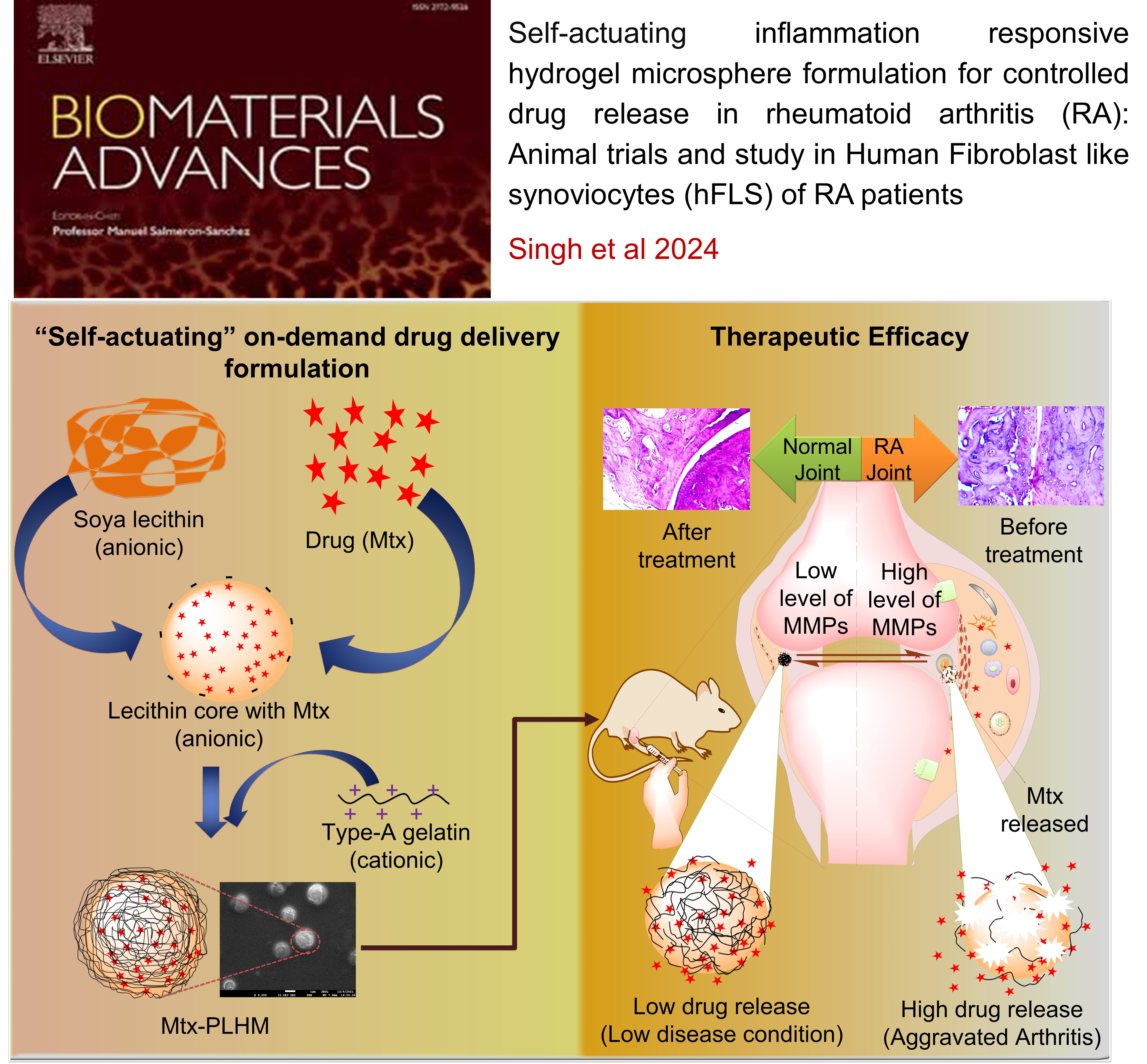
RHEUMATOID ARTHRITIS: Self-actuating inflammation responsive hydrogel microsphere formulation for controlled drug release in rheumatoid arthritis (RA) : Animal trials and study in human fibroblast like synoviocytes (hFLS) of RA patients
This study developed an intelligent, "self-actuating" drug delivery system for rheumatoid arthritis (RA) that releases the drug methotrexate (Mtx) in response to inflammation. The system uses MMP-responsive, polymer-lipid hybrid hydrogel microspheres (Mtx-PLHM) that are injected directly into the joints. These microspheres release Mtx only when the inflammatory enzymes MMP-2 and MMP-9, which are elevated in RA patients, are present. In a rat arthritic model, Mtx-PLHM showed significant therapeutic benefits by reducing joint inflammation, swelling, and bone erosion, with minimal systemic side effects. This targeted approach lowers the need for repeated joint injections and protects patients from unnecessary drug exposure when inflammation is not present.
-
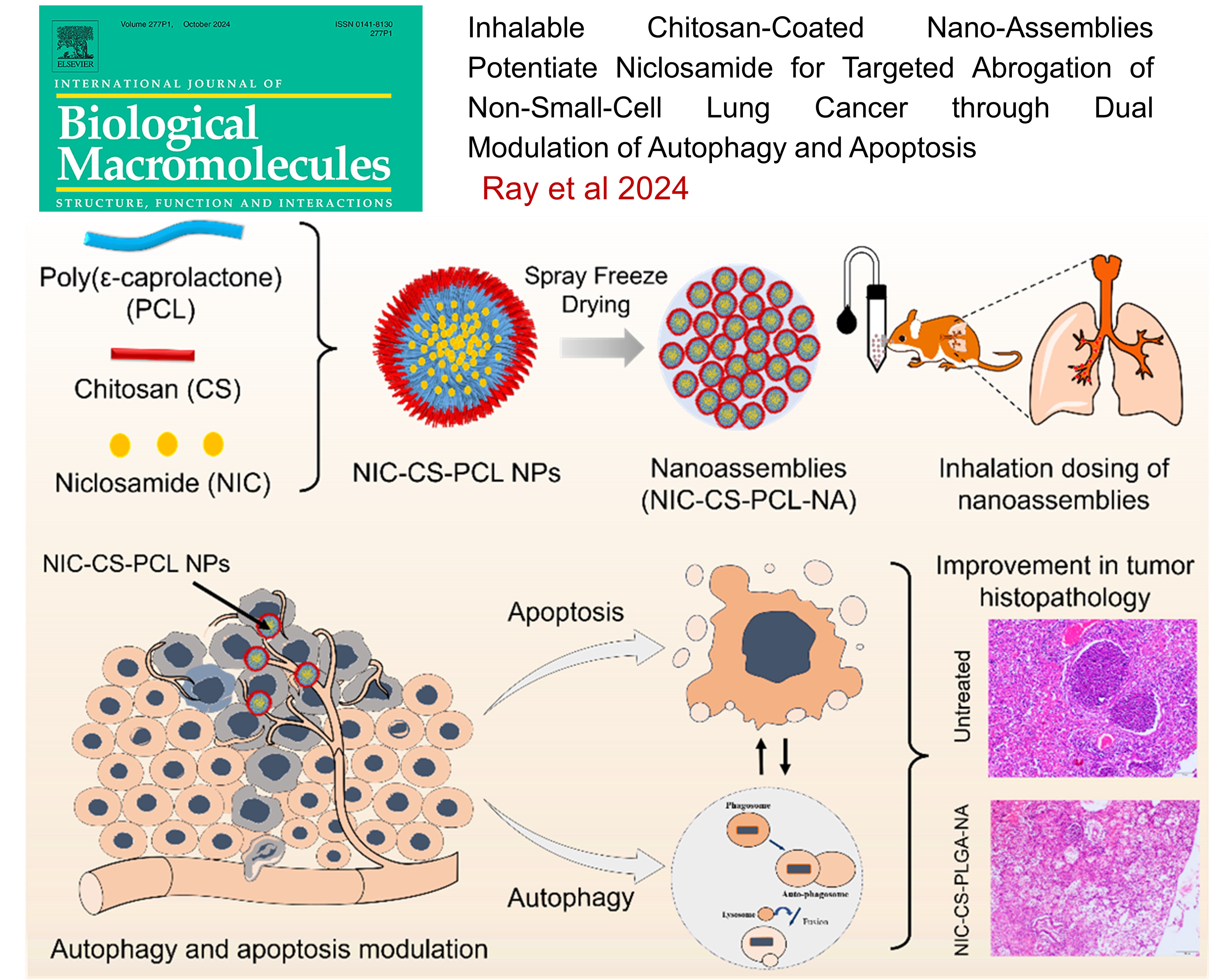
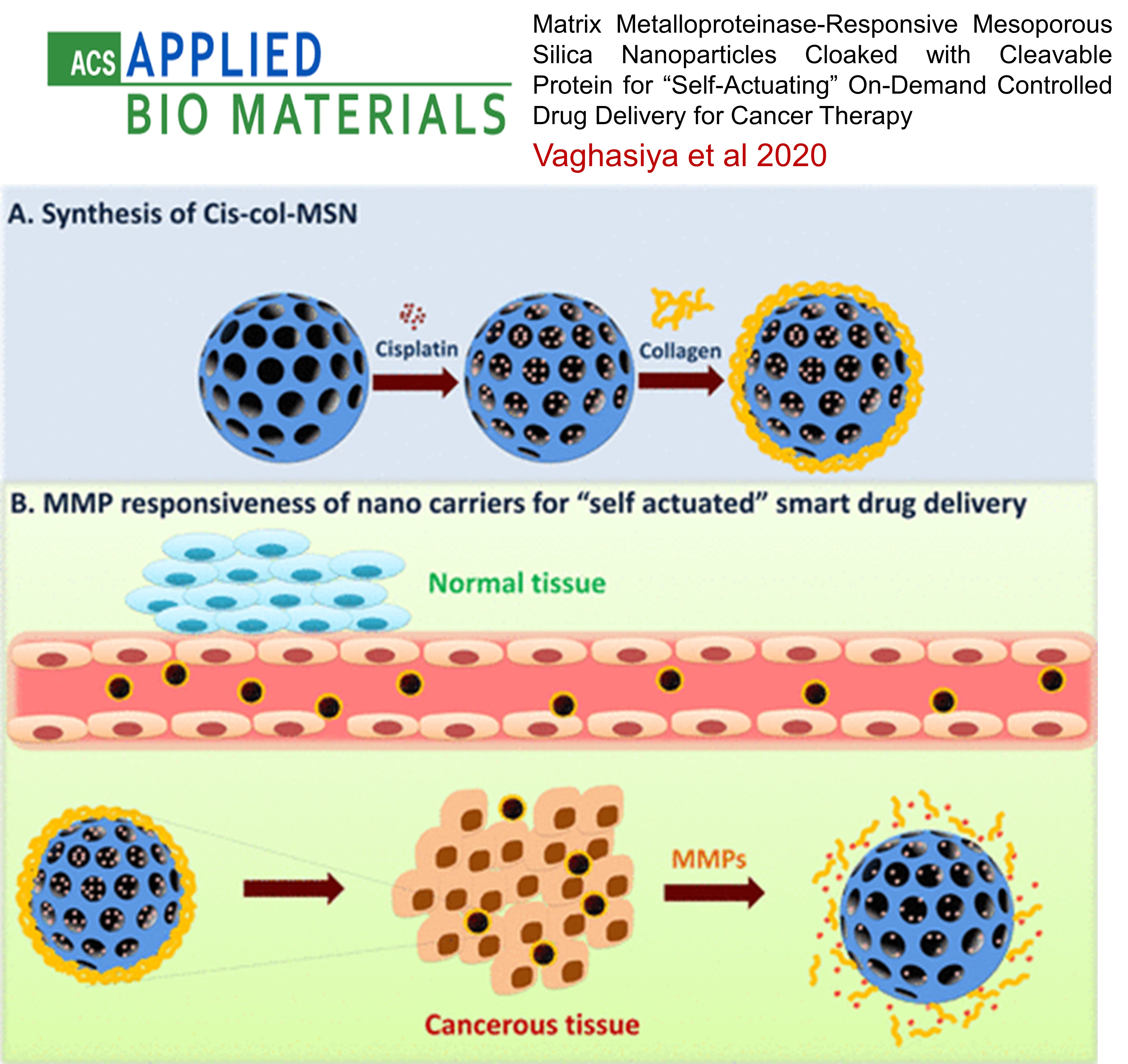
LUNG CANCER: Matrix Metalloproteinase-Responsive Mesoporous Silica Nanoparticles Cloaked with Cleavable Protein for “Self-Actuating” On-Demand Controlled Drug Delivery for Cancer Therapy
This study developed MMP-2-responsive mesoporous silica nanoparticles (MSNs) for targeted cancer therapy. Cisplatin-loaded MSNs were coated with collagen to prevent drug release under normal conditions. In the tumor microenvironment, overexpressed MMP-2 enzymes triggered the release of the drug by removing the collagen cap. The nanoparticles showed efficient uptake, biocompatibility, and increased cytotoxicity in lung cancer cells, enhancing therapeutic effects by promoting reactive oxygen species, cell cycle arrest, and apoptosis. This system offers a promising approach for "on-demand" drug delivery specifically at tumor sites
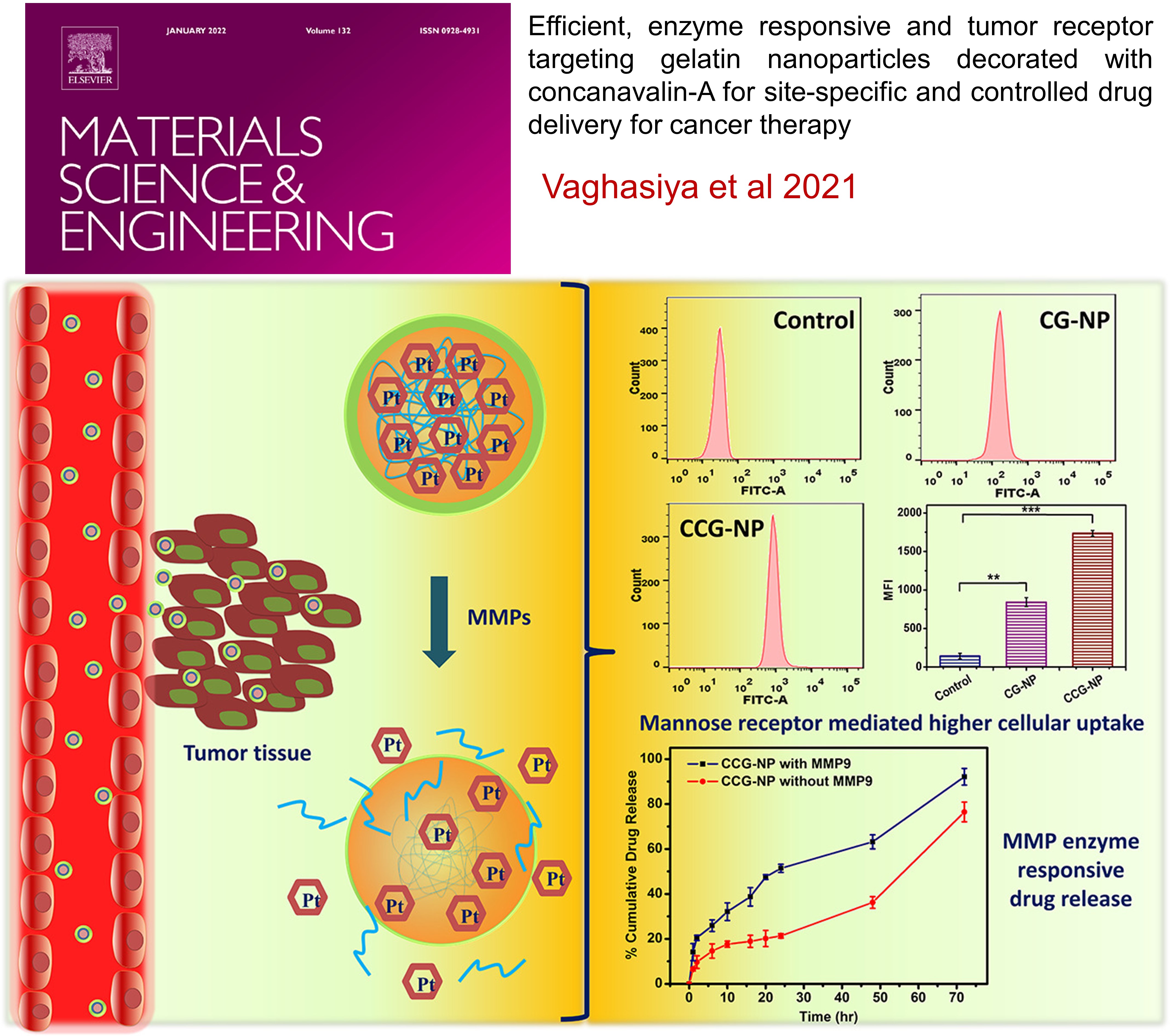
Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy
This study developed mannose receptor-targeted and MMP-responsive gelatin nanoparticles (CCG-NP) for lung cancer therapy. These smart nanoparticles are surface-decorated with concanavalin A (con-A) to enhance uptake by cancer cells, which have overexpressed mannose receptors. Cisplatin-loaded gelatin nanoparticles demonstrated enzyme-triggered drug release and increased cellular internalization in lung cancer cells. The system significantly enhanced reactive oxygen species generation, cell cycle arrest, and apoptosis in cancer cells. The inhalable CCG-NP offers a targeted and "on-demand" drug delivery system, ensuring effective therapeutic delivery at the tumor site while minimizing off-target toxicity.
Current Group Members
-

FARDEEN ALI
Email: fardeen.ar250802@inst.ac.in
Reg. No.: 10BB25A67002
-

NANDITA
Email: nandita.ph25210@inst.ac.in
Reg. No.: PH25210
-

DEEPTI SIWACH
Email: deepti.ph25205@inst.ac.in
Reg. No.: PH25205
-

SWARNIMA NEGI
Email: swarnima.ph22206@inst.ac.in
Reg. No.: PH22206
-

AGRIM JHILTA
Email: agrim.ph21232@inst.ac.in
Reg. No.: PH21232
-

RAGHURAJ SINGH
Email: raghuraj.ar200803@inst.ac.in
Reg. No.: 10BB20A67003
-

GOURAB DAS
Email: gourab.rp1522591@inst.ac.in
Reg. No.: RP1522591
-

AISHWARYA JAISWAL
Email:
Reg. No.:
Alumni
-

RASHMI GHOSH
Reg. No.: IN-2024/21
Designation: Intern Student
Jun 2024 - Jul 2024
-

JADHAV KRISHNA SUDHAKAR
Reg. No.: PH20225
Designation: PhD Scholar
Aug 2020 - Sep 2025
-

EUPA RAY
Reg. No.:
Designation: PhD Scholar
May 2016 - Apr 2023
-

KALPESH VAGHASIYA
Reg. No.:
Designation: PhD Scholar
Mar 2015 - Nov 2021
-

MR. ANKUR SHARMA
Reg. No.: PH14208
Designation: PhD Scholar
Aug 2014 - Aug 2020
-
1.
Engineered vildagliptin-loaded polymeric nanoparticles via microfluidic and spray drying for enhanced antidiabetic activity , E Kole, K Jadhav, Z Khan, RK Verma, A Chatterjee, A Mujumdar, J Naik, , Future Journal of Pharmaceutical Sciences , 2024 , 10 , 156 -
2.
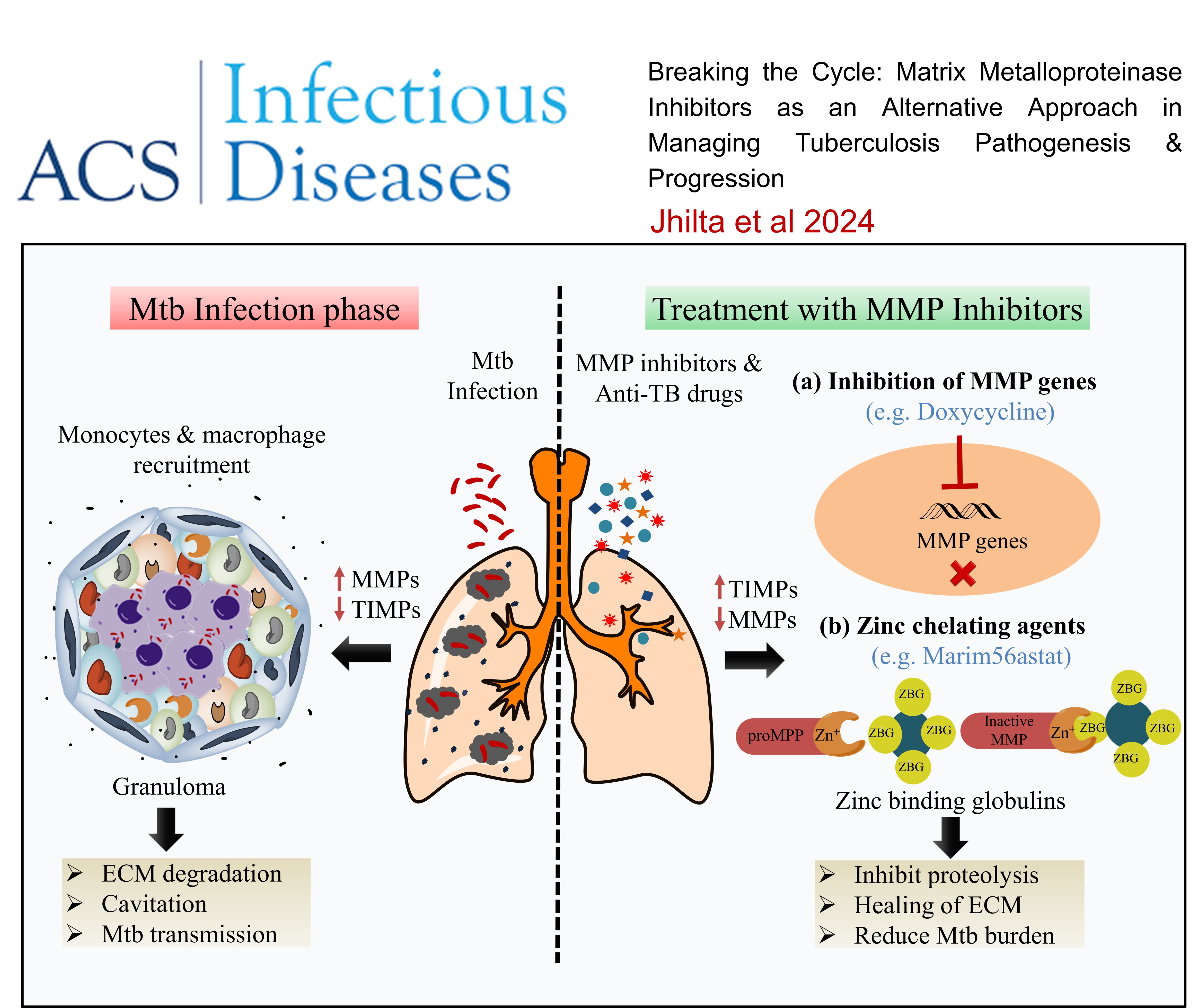
Breaking the Cycle: Matrix Metalloproteinase Inhibitors as an Alternative Approach in Managing Tuberculosis Pathogenesis and Progression , Agrim Jhilta, Krishna Jadhav, Raghuraj Singh, Eupa Ray, Alok Kumar, Amit Kumar Singh, Rahul Kumar Verma, , ACS Infectious Diseases , 2024 , 10 , 2567 -
3.
Targeted delivery of the metastasis-specific tumour Q2 homing TMTP1 peptide to non-small-cell lung cancer (NSCLC) using inhalable hybrid nano-assemblies , Eupa Ray, Krishna Jadhav, Monika Kadian, Garima Sharma,Kritika Sharma, Agrim Jhilta, Raghuraj Singh, Anil Kumar* and Rahul Kumar Verma* , Journal of Material chemistry-B , 2024 , 12 , 9740-9759 -
4.
Self-actuating inflammation responsive hydrogel microsphere formulation for controlled drug release in rheumatoid arthritis (RA , Raghuraj Singh, Krishna Jadhav, Rohit Kamboj, Hitesh Malhotra, Eupa Ray, Agrim Jhilta, Varun Dhir, Rahul Kumar Verma , Biomaterial Advances , 2024 , 160 , 213853 , 10.1016/j.bioadv.2024.213853 -
5.
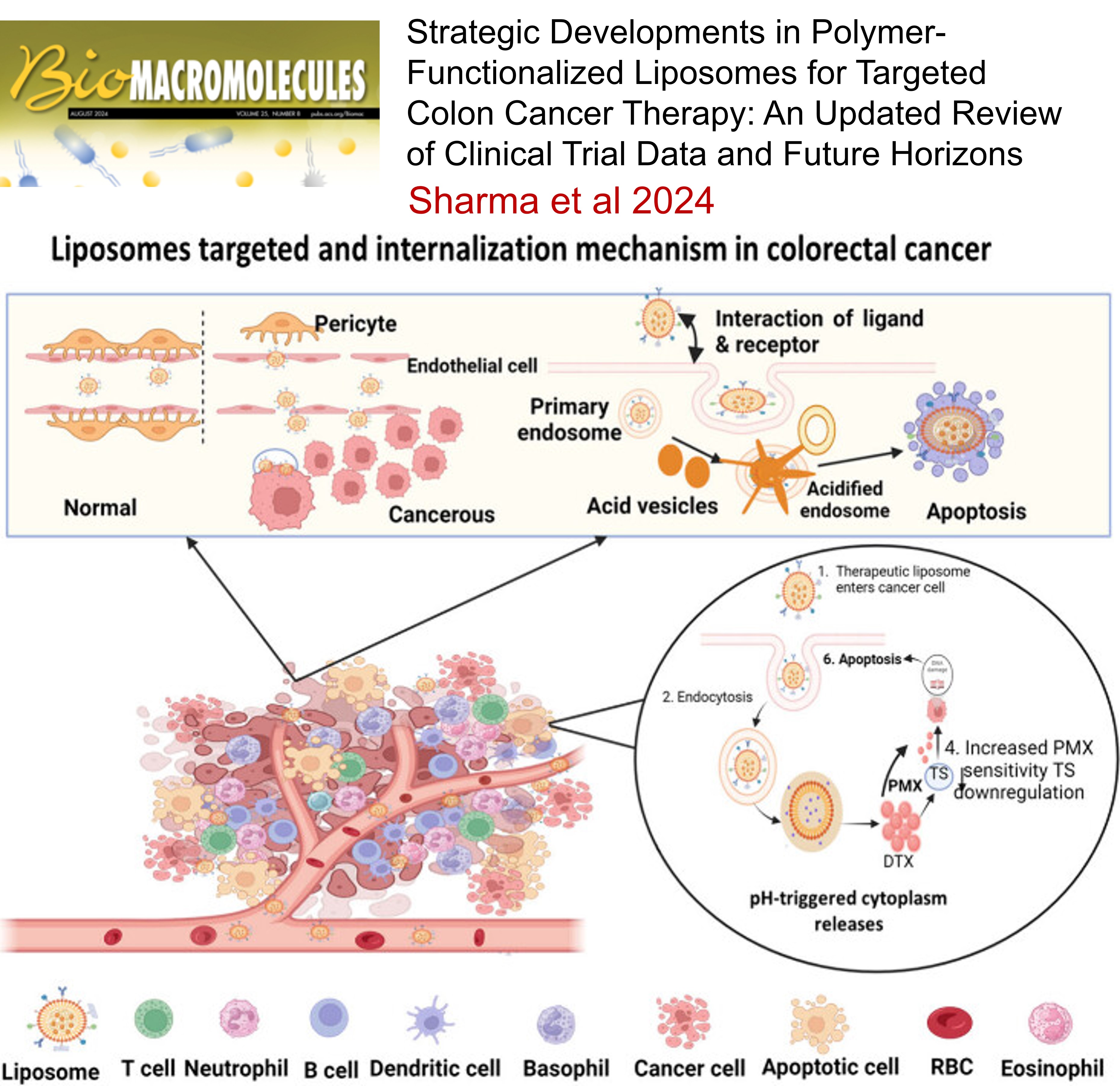
Strategic Developments in Polymer-Functionalized Liposomes for Targeted Colon Cancer Therapy: An Updated Review of Clinical Trial Data and Future Horizons , Satyam Sharma, Moitrai Chakraborty, Dharmendra Yadav, Aniket Dhullap, Raghuraj Singh, Rahul Kumar Verma, Sankha Bhattacharya, Sanjiv Singh , Biomacromolecules , 2024 , 145 , 985 -
6.
Quality by Design in Pulmonary Drug Delivery: A Review on Dry Powder Inhaler Development, Nanotherapy Approaches, and Regulatory Considerations , Ashish Dilip Sutar, Rahul Kumar Verma, Rahul Shukla , AAPscitech , 2024 , 78 , 125 , https://doi.org/10.1208/s12249-024-02900-z -
7.
Recent Developments in Tyrosine Kinase Inhibitor-based Nanotherapeutics for EGFR-resistant Non-small Cell Lung Cancer , Eknath Kole, Krishna Jadhav, Raghuraj Singh, Shilpa Mandpe, Ashwin Abhang, Rahul K Verma, Jitendra Naik, , Current drug Delivery , 2024 , Accepted -
8.
A critical review on developments in drying technologies for enhanced stability and bioavailability of pharmaceuticals , Krishna Jadhav, Eknath Kole, Raghuraj Singh, Saroj Kumar Rout, Rahul Kumar Verma, Aniruddha Chatterjee, Arun Mujumdar, Jitendra Naik , Drying Technology , 2024 , 9 , 1415-1441 , https://doi.org/10.1080/07373937.2024.2357181 -
9.
Recent Advances in Targeting Transition Metals (Copper, Iron, and Zinc) in Alzheimer’s Disease , Raghuraj Singh, Archna Panghal, Krishna Jadhav, Ashima Thakur, Rahul Kumar Verma, Charan Singh, Manoj Goyal, Jayant Kumar, Ajay G Namdeo, , Molecular Neurobiology , 2024 , Accepted , 10.1007/s12035-024-04256-8 -
10.
Effective Cerebral Tuberculosis Treatment via Nose-to-Brain Transport of Anti-TB Drugs Using Muco-Adhesive Nano-aggregates , Krishna Jadhav, Agrim Jhilta, Raghuraj Singh, Vimal Kumar, Awadh Yadav, Amit Kumar Singh, Rahul Kumar Verma, Eupa Ray , Nanoscale , 2024 , 23 , 213594 -
11.
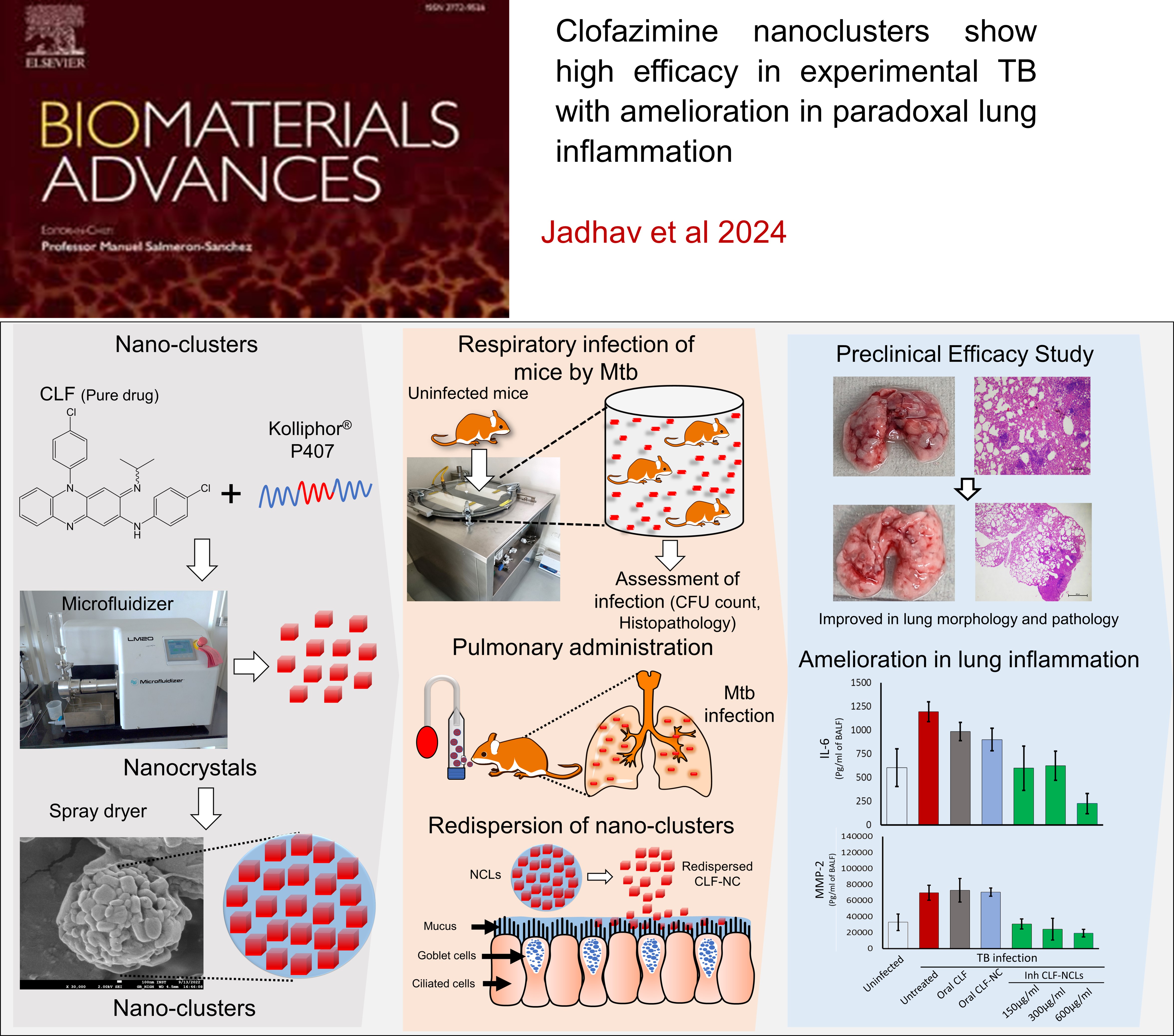
Clofazimine nanoclusters show high efficacy in experimental TB with amelioration in paradoxical lung inflammation , K Jadhav, A Jhilta, R Singh, E Ray, N Sharma, R Shukla, AK Singh, RK Verma , Biomaterials Advances , 2024 , 153 , 213594 , 10.1016/j.bioadv.2023.213594 -
12.
Inhalable Chitosan-Coated Nano-Assemblies Potentiate Niclosamide for Targeted Abrogation of Non-Small-Cell Lung Cancer through Dual Modulation of Autophagy and Apoptosis , Eupa Ray1,2, Krishna Jadhav1, Monika Kadian2, Garima Sharma2, Kritika Sharma2, Agrim Jhilta1, Raghuraj Singh1, Anil Kumar2#, and Rahul Kumar Verma1# , International Journal of Biological Macromolecules , 2024 , 279 , 135411 -
13.
Transnasal brain delivery of anti-TB drugs by methyl-β-cyclodextrin microparticles show efficient mycobacterial clearance from central nervous system , Krishna Jadhav, Agrim Jhilta, Raghuraj Singh, Shweta Sharma, Swarnima Negi, Kailash Ahirwar, Rahul Shukla, Amit Kumar Singh, Rahul Kumar Verma , Journal of controlled release , 2024 , Accepted -
14.
New Generation Smart Drug Delivery Systems for Rheumatoid Arthritis , R Singh, K Jadhav, K Vaghasiya, E Ray, R Shukla, RK Verma , Current Pharmaceutical Design , 2023 , 29 (13) , 984-1001 , http://dx.doi.org/10.2174/1381612829666230406102935 -
15.
Harnessing personalized tailored medicines to digital-based data-enriched edible pharmaceuticals, , M Handa, O Afzal, S Beg, S Nasik, RK Kaundal, RK Verma, R Shukla* , Drug Discovery Today , 2023 , 5 , 103555 , 10.1016/j.drudis.2023.103555 -
16.
Fat fighting liraglutide based nano-formulation to reverse obesity: Design, development and animal trials , DK Jakhar, VK Vishwakarma, R Singh, K Jadhav, S Shah, T Arora, RK Verma* and HN Yadav* , International Journal of Pharmaceutics , 2023 , 634 , 122585 -
17.
Design, Synthesis, Molecular docking, and Antibacterial Study of Aminomethyl Triazolo Substituted Analogues of Benzimidazolo [1, 4]-benzodiazepine , M Devi, S Jaiswal, N Yaduvanshi, S Jain, S Jain, K Verma, RK Verma, D Kishore, J Dwivedi, S Sharma, , Journal of Molecular Structure , 2023 , 1286 , 135571 , https://doi.org/10.1016/j.molstruc.2023.135571 -
18.
Comparative Preclinical Pharmacokinetics and Disposition of Favipiravir Following Pulmonary and Oral Administration as Potential Adjunct Therapy Against Airborne RNA Viruses , Venkata Siva Reddy Devireddy, Hasham Shafi, Sonia Verma, Sanjay Singh, JVUS Chakradhar, Naresh Kothuri, Himanshu Bansode, Sunil Kumar Raman, Deepak Sharma, Lubna Azmi, Rahul Kumar Verma, Amit Misra , Pharmaceutical Research , 2014 , 5 , 1-10 -
19.
Taming the devil: Antimicrobial peptides for safer TB therapeutics: , Krishna Jadhav,Raghuraj Singh,Eupa Ray,Amit Singh,Rahul K Verma , (2022) , (Accepted) , Current Protein & Peptide Science -
20.
Formulation and Optimization of Silymarin Encapsulated Binary Micelles for Enhanced Amyloid Disaggregation Activity: , Ajit Singh, Rewati Raman Ujjwal , Ashish Kumar , Rahul K Verma , Rahul Shukla , (2022) , 28: 1-35 , Drug Development and Industrial Pharmacy , 10.1080/03639045.2022.2059498 -
21.
Formulation development of tocopherol polyethylene glycol nanoengineered polyamidoamine dendrimer for neuroprotection and treatment of alzheimer disease: , Ajit singh, Rewati raman, Rahul Verma, Sanjay Tiwari, Prashant keserwani, Rahul Shukla , (2022) , 18: 1-15. , Journal of Drug Targeting ( Accepted) , doi: 10.1080/1061186X.2022.2063297 -
22.
Pain allaying epalrestat-loaded lipid nanoformulation for the diabetic neuropathic pain interventions: Design, development and animal study: , Vishal Viswakarma, Dheeraj Jhakkar, Rahul K Verma*, Harlokesh Yadav*, , (2022) , (Accepted): , Current Drug Metabolism -
23.
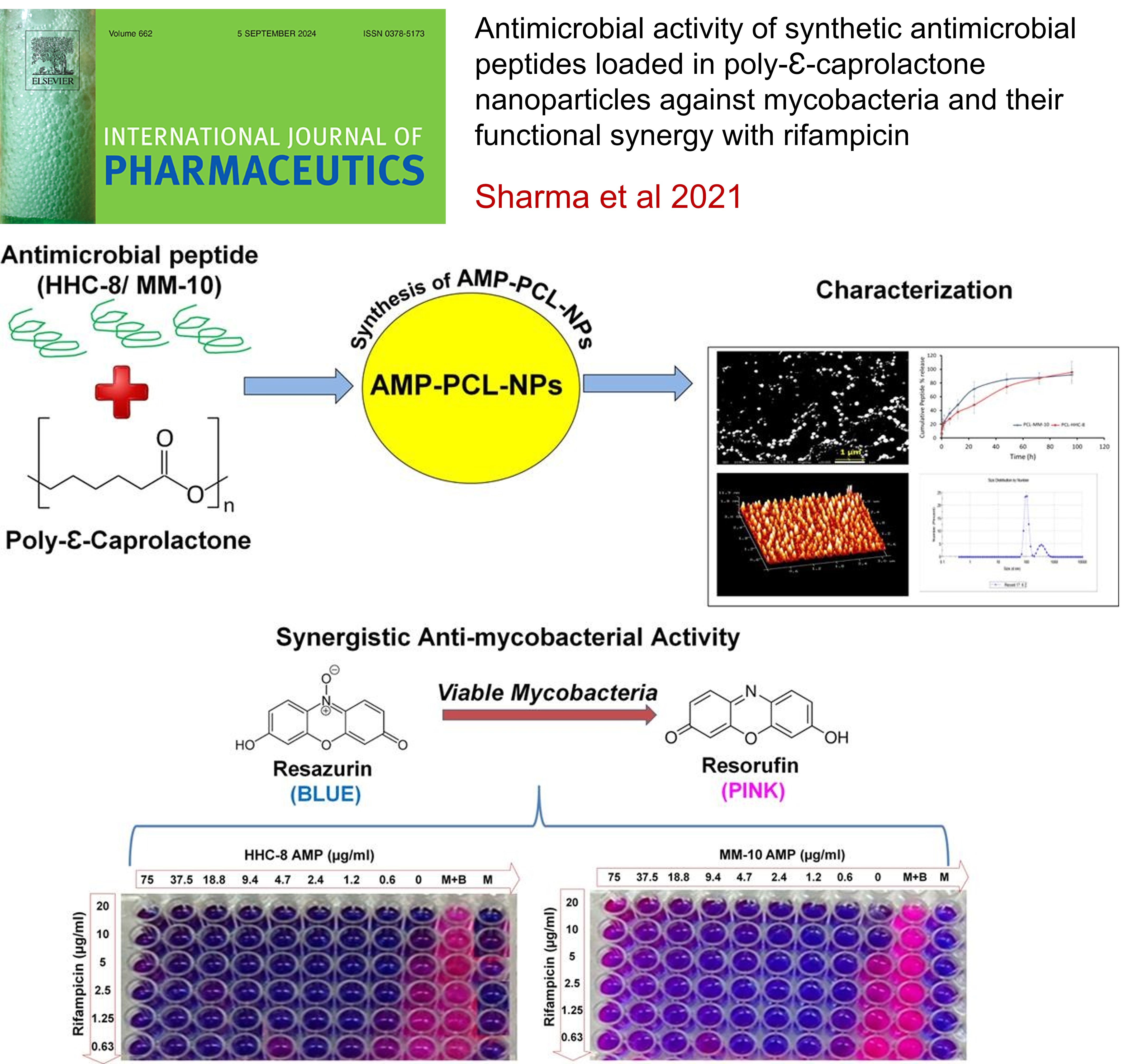
Antimicrobial Activity of Synthetic Antimicrobial Peptides Loaded in Poly-Ɛ-Caprolactone Nanoparticles Against Mycobacteria and their Functional Synergy With Rifampicin: , Ankur Sharma,Aparna Gaur,Neelesh Sharma,Rahul K Verma*,Amit K Singh* , (2021) , 14: 121097 ( Impact Factor-5.88) , International Journal of Pharmaceutics , 10.1016/j.ijpharm.2021.121097 -
24.
Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy: , Kalpesh Vaghasiya, Eupa ray,Raghuraj singh,Krishna Jadhav,Ankur Sharma,Rehan Khan,Rahul Kumar Verma , (2021) , 123: 112027 (Impact factor-7.32) , Materials Science and Engineering: C , https://doi.org/10.1016/j.msec.2021.112027 -
25.
Systematic development and optimization of spray-dried Quercetin-HP-β-Cyclodextrin microparticles for DPI-based therapy of lung cancer: , Kalpesh Vaghasiya,Eupa Ray ,Ankur Sharma ,Raghuraj Singh ,Rehan Khan,Om Prakash Katare,Rahul Kumar Verma* , (2021) , 56: 14700–14716 (Impact factor-4.22) , Journal of Materials Science , https://link.springer.com/article/10.1007/s10853-021-06205-5 -
26.
Enema Based Therapy Using Liposomal Formulation of Low Molecular Weight Heparin for Treatment of Active Ulcerative Colitis: New Adjunct Therapeutic Opportunity : , Anas Ahmad# , Kalpesh Vaghasiya#, Ajay Kumar , Pravej Alam, Syed Shadab Raza, Rahul Kumar Verma*, Rehan Khan* , (2021) , 121: 111851 (Impact factor-7.32) , Materials Science and Engineering: C , https://doi.org/10.1016/j.msec.2020.111851 -
27.
Inhalable Particles Containing Isoniazid and Rifabutin as Adjunct Therapy for Safe, Efficacious and Relapse-Free Cure of Experimental Animal Tuberculosis in One Month: , Amit singh, Rahul Kumar Verma,Amit Misra , (2021) , 128: 102081 (impact factor-3.13) , Tuberculosis , https://doi.org/10.1016/j.tube.2021.102081 -
28.
Inhalation delivery of host defence peptides (HDP) using nano-formulation strategies: A pragmatic approach for therapy of pulmonary ailments : , Adlakha S, Sharma A, Vaghasiya K, Ray E,Verma RK* , (2020) , 21: 369 - 378 (IF:2.37) , Current Protein & Peptide Science , 10.2174/1389203721666191231110453 -
29.
Dynamic mucus penetrating microspheres for efficient pulmonary delivery and enhanced efficacy of host defense peptide (HDP) in experimental tuberculosis: , Sharma A,Vaghasiya K,Gupta P,Singh AK,Gupta UD,Verma RK* , (2020) , 324: 17-33 (Impact factor-9.77) , Journal of Controlled Release , https://doi.org/10.1016/j.jconrel.2020.05.013 -
30.
Enteric-Coated Gelatin Nanoparticles Mediated Oral Delivery of 5-Aminosalicylic Acid Alleviates Severity of DSS-Induced Ulcerative Colitis: , Anas Ahmad, Md. Meraj Ansari, Rakesh Kumar Mishra, Ajay Kumar, Akshay Vyawahare, Rahul Kumar Verma, Syed Shadab Raza, Rehan Khan* , (2020) , 119: 111582 (Impact factor-7.32) , Materials Science and Engineering: C , 10.1016/j.msec.2020.111582 -
31.
Autophagy inducing inhalable Co-crystal formulation of Niclosamide-Nicotinamide for lung cancer therapy: , Ray E, Vaghasiya K, Sharma A, Shukla R, Khan R, Verma RK* , (2020) , 21: 260 , AAPS PharmSciTech , https://link.springer.com/article/10.1208/s12249-020-01803-z -
32.
Targeted pulmonary delivery of Epigallocatechin gallate (EGCG), a green tea polyphenol controls the growth of Mycobacterium tuberculosis by enhancing the autophagy and supressing bacterial bu: , Sharma A,Vaghasiya K,Ray E,Gupta P,Gupta UD,Singh AK,Verma RK* , (2020) , 6: 4126–4140 (IF:4.51) , ACS Biomaterials Science & Engineering , https://doi.org/10.1021/acsbiomaterials.0c00823 -
33.
Matrix metalloproteinase responsive mesoporous silica nanoparticles cloaked with cleavable-protein for “Self-actuating” on-demand controlled drug delivery for cancer therapy: , Vaghasiya K, Ray E, Sharma A, Katare OP, Verma RK* , (2020) , 3: 4987–4999 , ACS Applied Bio Materials , https://doi.org/10.1021/acsabm.0c00497 -
34.
In vitro anti-tumoral and anti-bacterial activity of octamolybdate cluster-based hybrid solid incorporated with copper picolinate complex: , Arti Joshi,Ruby Gupta, Kalpesh Vaghasiya,Rahul Kumar Verma,Deepika Sharma,Monika Singh* , (2020) , 3: 4025–4035 , ACS Applied Bio Materials , https://doi.org/10.1021/acsabm.0c00093 -
35.
Silymarin encapsulated nanoliquid crystals for improved activity against beta amyloid induced cytotoxicity: , Singh A, Kumar A, Verma RK, Shukla R , (2020) , 7;149: 1198-1206 (IF:4.8) , International Journal of Biological Macromolecules , 10.1016/j.ijbiomac.2020.02.041. -
36.
Heparin encapsulated metered-dose topical “Nano-spray gel” liposomal formulation ensures rapid on-site management of frostbite injury by inflammatory cytokines scavenging: , Vaghasiya K, Sharma A, Kumar K, Ray E, Adhlakha S, Katare OP, Hota S,Verma RK* , (2019) , 5: 6617-6631 (IF:4.51) , ACS Biomaterials Science & Engineering , https://doi.org/10.1021/acsbiomaterials.9b01486 -
37.
Polycaprolactone based nano-carrier for co-administration of moxifloxacin and rutin and its in-vitro evaluation for sepsis: , Handa M, Sharma A,Verma RK,Shukla R , (2019) , 56: 101286 (IF:2.60) , Journal of Drug Delivery Science and Technology , 10.1016/j.jddst.2019.101286 -
38.
Alginate Micro-Spheres elicit innate Th1-inflammatory response in macrophages leading to bacillary killing: , Vaghasiya K, Eram A, Sharma A, Ray E, Adlakha S,Verma RK* , (2019) , 20(6): 241 (IF:2.82) , AAPS PharmSciTech , 10.1208/s12249-019-1458-0 -
39.
Temperature/pH triggered PNIPAM based Smart nanogel system loaded with Anastrozole delivery for application in cancer chemotherapy AAPS PharmScitech: , Handa M, Sharma A,Verma RK,Shukla R , (2019) , 20(5): 213 (IF:2.82) , AAPS PharmSciTech , 10.1208/s12249-019-1410-3 -
40.
Nanostructured silver decorated hollow silica and their application in treatment of microbial contaminated water at room temperature: , Baruah A, Vaghasiya K, Ganguli AK,Verma RK ,Jha M , (2019) , 458(1): 84-89 (IF:3.06) , New Journal of Chemistry , 10.1039/C9NJ01049A
-
1.
Methods to Characterize Nanoparticles for Mucosal Drug Delivery: , Kalpesh Vaghasiya Ankur Sharma Eupa Ray Suneera Adlakha Rahul Kumar Verma , (2020) , Mucosal Delivery of Drugs and Biologics in Nanoparticles, Kalpesh Vaghasiya Ankur Sharma Eupa Ray Suneera Adlakha Rahul Kumar Verma -
2.
Inhalable polymeric dry powders for antituberculosis drug delivery: , Suneera Adlakha,Kalpesh Vaghasiya,Ankur Sharma,Eupa Ray,Rahul Kumar Verma , (2020) , Nanotechnology based approaches for tuberculosis treatment, Elsevier -
3.
Molecular Medicines for Cancer: Concepts and Applications of Nanotechnology CRC Press/Taylor & Francis Publishers: , Ray E, Sharma A, Vaghasiya K , (2018) -
4.
DNA nanostructures. chemistry, self-assembly and applica-tion, Emerging Applications of Nanoparticles and Architecture Nanostructures, Springer: , Sharma A, Vaghasiya K, Yadav AB , (2018) -
5.
Lung anatomy and physiology and their implications for pul-monary drug delivery, Edited by-Nokhodchi and Martin, Pulmonary drug delivery: Advances and challenges, Wiley press, USA: , Ibrahim M,Garcia-Contreras L. , (2015) -
6.
Modulation of host pathogen inter-action following particle phagocytosis by infected macrophages In: Norazmi Mohd Nor, Armando Acosta and Maria Elena Sarmiento (Eds). The Art and Science of T: , Singh Ak, Gupta A, Mohan M, Agrawal AK, Sharma R, Misra A , (2014)
-
1.
Hybrid Nano-in-Micro systems for lung delivery of Host Defence Peptides (HDP) as ad-junct therapeutics for Pulmonary TB: , Drug Delivery to Lungs Edinburg , (2017) -
2.
Hybrid Nano-in-Micro systems for lung delivery of Host Defence Peptides (HDP) as ad-junct therapeutics for Pulmonary TB: , Sharma A, Verma RK , (2017) , 3: 113–115 , Drug Delivery to Lungs -
3.
Saying NO (Nitric Oxide) to Tuberculosis: , INHALATION ASIA , (2015) -
4.
Lung Delivery of Anti-microbial Peptides (AMP) Using Dry Powder Inhalable Micro-spheres for Treatment of Pulmonary Tuberculosis: , Respiratory drug delivery , (2015) -
5.
Augmenting the macrophage nitric oxide response to intracellular Mycobacterium tuberculosis. Conference on Inhaled therapy for tuberculosis: , Misra A , (2013) -
6.
Gupta P Quantitative PCR for persistent bacterial DNA following pulmonary delivery of microparticles Conference on Inhaled therapy for tuberculosis: , Singh Ak, Mohan M, Agrawal AK, Misra A, Gupta UD , (2013) -
7.
Development and validation of a HPLC method to determine concentrations of a novel anti-cancer compound, DT330 in biological samples AAPS: , Raghuvanshi D, Bailey-Downs LC, Kunch A, Ihnat MA, and Garcia-Contreras L , (2013) -
8.
Optimization of Spray Freeze Drying Process Conditions to Prepare West Nile Virus Vaccine for Nasal Immunization AAPS: , I brahim, Raghuvanshi D, Abraham SN, Staats HF, and Garcia-Contreras L , (2013) -
9.
Nitric oxide releasing PLGA nanoparticles: evaluation of antileishmanial activity. NanoBio-2012 Second International Conference on Nanotechnology and Molecular Medicine at Biomedical interfa: , Pandya S, Khare P, Dube A, Verma PRP,Misra A , (2012) -
10.
Preclinical safety, efficacy and mechanism of action of inhaled microparticles containing anti-tuberculosis agents. Third indo-Japanese International Joint Symposium on overcoming interactabl: , Sharma R, Muttil P, Yadav AB, Singh AK, Devi HK, Mohan M, Arora SK, Sen H, Sinha S, Suryakumar J, Modak V, Vineeth R , (2010) -
11.
The Academy of Pharmaceutical Sciences of Great Britain, University of Nottinghum, Nottinghum, UNITED KINGDOM(UK) (attended): , Pharm Science , (2010) -
12.
Inhalable poly lactide particles as drug carrier against tuberculosis (Oral presentation). NIPER Second Winter school 2009–Nanotechnology in advance Drug delivery, National Institute of Phar: , Misra A , (2009) -
13.
Serendipitous activation of mouse and human macrophages infected with Mycobacterium tuberculosis on treatment with inhalable microparticles. An International Symposium on -Optimizations of I: , Sharma R, Muttil P, Yadav AB, Singh AK, Mohan M, Misra A , (2009) -
14.
The devil's advocacy: When and why inhaled therapies for tuberculosis may not work. A International Symposium on -Optimizations of Inhaled Tuberculosis Therapies and Implications for Host-Pat: , Misra A, Yadav AB, Singh AK, Mohan M , (2009) -
15.
Microparticles specific for lung delivery: in-vivo drug distribution in monkeys. XVII International conference on Bioencapsulation-2009,Groningen, THE NETHERLANDS: , Misra A. , (2009) -
16.
Serum, tissue and intracellular concentrations following pulmonary delivery in rhesus macaques (Oral) International Symposium on -Optimizations of Inhaled Tuberculosis Therapies and Implicat: , Misra A. , (2009) -
17.
Inhalable poly lactide particles as drug carrier against tuberculosis (Oral presentation). NIPER Second Winter school 2009–Nanotechnology in advance Drug delivery, National Institute of Phar: , Misra A , (2009) -
18.
Pharmacokinetic of PLA microparticles in Rhesus monkeys. XVI International conference on Bioencapsulation-2008, Dublin, IRELAND : , Kaur J, Yadav AB, Kumar K, Misra A. , (2008) -
19.
PLA microparticles for pulmonary delivery of AntiTB drugs: biodistribution study. XVI International conference on Bioencapsulation-2008,Dublin, IRELAND: , Kaur J, Yadav AB, Kumar K,Misra A. , (2008) -
20.
Inhalable particles for value added drug delivery in tuberculosis: Singh AK, Heikham KD, Mohan M, Agrawal AK, Gupta A, (2011) INDO-US joint Symposium on Nanomedicine: , Propects and Challenges
Senior Research Fellow:Central Drug Research Institute (CDRI),, Lucknow, India (August 2009 to February 2014 )
Visiting fellow:Bradford University, UK (May 2010 to August 2010 )
Scientist-C:Institute of Nano Science and Technology (INST), Mohali,, Punjab, India (April 2014 to April 2017 )
Scientist-D:Institute of Nano Science and Technology (INST), Mohali,, Punjab, India (January 2018 to December 2020 )
Scientist-E:Institute of Nano Science and Technology (INST), Mohali, India (January 2021 to Present till date )
Visiting Scientist- Harvard Medical School, USA ( March 2023 to February 2024)
Member of Royal Society of Biology, United Kingdon (UK)
DHR-ICMR International Fellow
Elected Member of National Academy of Medical Sciences (MAMS), India
Royal Society of United Kingdom Fellowship UK- CSIR, INDIA-2009-10 – received to work in UK at Institute of pharmaceutical Innovation (IPI) Bradford University in year 2009-10.
Director’s Special CDRI incentive award-2008.
Bioencapsulation Research Group (BRG)- France travel Award 2009- received to travel and present at Groningen University, THE NETHERLANDS
Bioencapsulation Research Group (BRG)- France travel Award 2008- received to travel and present at Dublin City University, Dublin, IRELAND.
CSIR-SRF – Qualified Senior Research Fellowship-2009 (Council of Scientific & Industrial Research (CSIR), INDIA.
Post Graduate fellowship from NIPER during MS (Pharm) course (2003-2005)
GATE (Graduate Aptitude Test for Engineering) - Qualified in Pharmaceutical Sciences -2004.organized by Indian Institute of Technology (IIT), INDIA.
-
Title: Biodegradable multi-unit intra-ruminal devices for long-term time-controlled pulsatile release of nutrients supplements & de-worming drugs to enhance production and reproduction in cattle
PI: Dr. Rahul K verma
Funding Amount: 60 lakhs
Tenure: 3 Years
End Date: 31-Aug-2026
Funding Agency: DBT
-
Title: Self-titrating inflammation-responsive injectable smart nano-formulations for repetitive delivery of DMARDs and corticosteroids against Rheumatoid Arthritis
PI: Dr. Rahul K. Verma
CO-PI: Dr. Rehan Khan
Funding Amount: 41 lakhs
Tenure: 3 years
Funding Agency: DST-SERB(CRG )
-
Title: Enzyme activated targeted nano-formulations of autophagy/apoptosis-inducing bioactive as potential therapy for drug resistant lung cancer
PI: Dr. Rahul K. Verma
Funding Amount: 58 Lakhs
Tenure: 2 years
Funding Agency: Nanomission
-
Title: Pulmonary delivery of Host Defense Peptides (HDP) using Porous Nanoparticle-Aggregate Particles (PNAPs) for alveolar macrophage targeting in pulmonary tuberculosis
PI: Dr. Rahul K. Verma
Funding Amount: 27 lakhs
Tenure: 3 years
Funding Agency: DST-SERB(YSS)
-
Title: Metered dose transdermal Nano-Spray herbal gel formulation for rapid relief and effective management of cold injury at extreme altitudes
PI: Dr. Rahul K. Verma
Funding Amount: 9.95 lakhs
Tenure: 2 years
Funding Agency: CARS(DIHAR-DRDO)
-
Title: Development of strategies to make Host Defence Peptides pragmatically applicable for clinical use as adjunct host directed therapy against drug sensitive and resistant tuberculosis
PI: Dr. Rahul K Verma
Tenure: 3 years
Funding Agency: ICMR