Faculty Directory
Prof. Sharmistha Sinha
Professor, Scientist-F
At the Sinha Lab, we investigate how failures in biomolecular self-assembly and cellular organization drive some of the most urgent biomedical challenges of our time—cancer and antibiotic resistance. We focus on the molecular logic that governs protein organization and how its breakdown fuels disease. In cancer, we dissect the misregulation and aggregation of tumor suppressors such as p53, revealing how structural disorder and aberrant assembly rewire cellular decision-making and promote malignancy. In parallel, we probe how pathogenic protein assemblies and stress-adaptive biomolecular condensates enable microbial survival under antibiotic pressure, contributing to the global crisis of drug resistance. Our research bridges molecular chaos and functional design. By integrating quantitative biophysics, genetic engineering, super-resolution microscopy, and molecular design, we uncover the physical principles that dictate cellular order—how proteins fold, assemble, misassemble, and reorganize in space and time. We then repurpose these principles to engineer bio-nano machines: programmable protein compartments, synthetic nanoreactors, and smart intracellular delivery systems capable of targeted catalysis, precision drug release, and adaptive therapeutic responses. These bio-inspired nanobiomachines offer new strategies to combat cancer progression and to overcome antibiotic resistance through spatially controlled, multi-modal interventions. Beyond discovery, the Sinha Lab is a training ground for independent, interdisciplinary scientists driven to translate fundamental biology into transformative technologies. We cultivate a collaborative and inclusive environment that values intellectual rigor, creativity, and bold thinking. Trainees are encouraged to work across scales—from atomic structures to living systems—and to design solutions that merge biology, physics, and engineering. With strong emphasis on global exposure, scientific communication, translational innovation, and career development, the lab prepares researchers to lead in academia, biotechnology, and the emerging frontier of bio-nano engineering. If you are excited about decoding protein disorder, confronting cancer and antibiotic resistance, and building the next generation of nanobiomachines from the principles of life itself, we would love to hear from you.
Contact Information :
-
Email:
sinhas@inst.ac.in -
Personal Webpage:
Personal Webpage -
Google Link:
Google Link
-
-
Neurodegenerative diseases – Parkinson’s Disease
Investigating the molecular underpinnings of Parkinson’s, including protein aggregation, mitochondrial dysfunction, and neuroinflammation, using integrative structural and cellular biology approaches. -
Cancer biology and targeted therapies
Studying aberrant signaling pathways and protein complexes in cancer, with a focus on exploiting structural vulnerabilities for therapeutic development. -
Compartmentalization in cellular systems (e.g., cancer and neurodegeneration)
Exploring how cells use spatial organization—through organelles, protein scaffolds, and membraneless compartments—to regulate complex processes in disease contexts.
-
-
p53, Cancer and Chemoresistance
At the Sinha Lab, we’re obsessed with the misbehaving molecules that drive disease. Our main character? p53, the so-called “guardian of the genome”—until it mutates and turns rogue in cancer. We focus on a hotspot: the R273 mutation. But here’s the catch—not all R273 mutants act the same. [R273H] stays relatively stable, [R273C] forms neat amyloid clumps via disulfide bonds, and [R273L]? It’s the wild one, aggregating through hydrophobic chaos. Using biophysics, simulations, and imaging, we’ve decoded how these mutants destabilize, misfold, and contribute to different cancer outcomes.
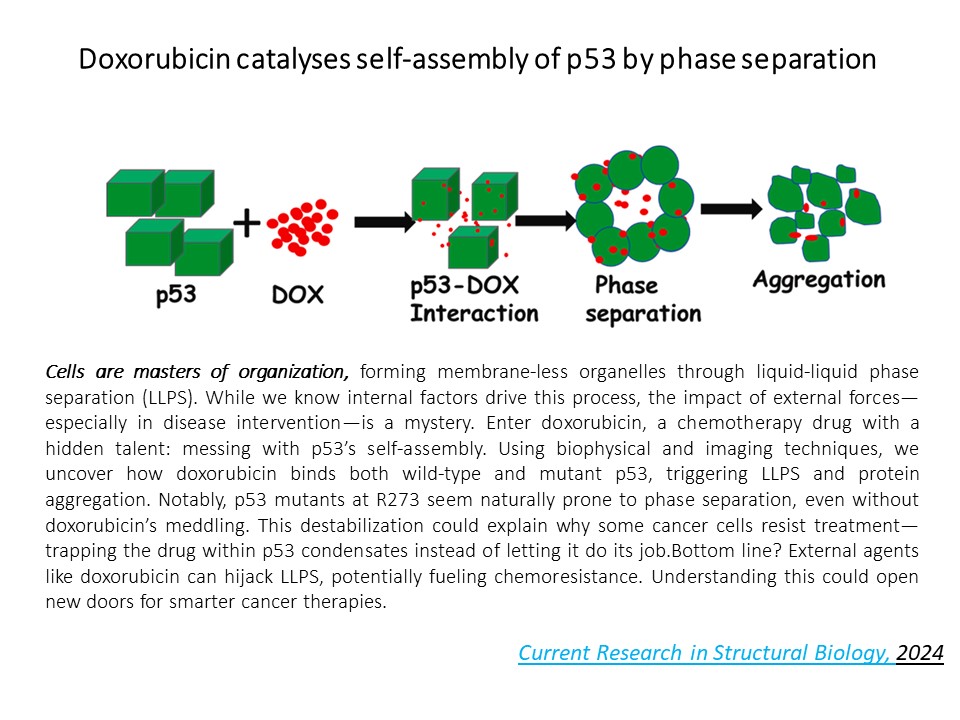
But it doesn’t stop there. Even wild-type p53 isn’t off the hook. When exposed to doxorubicin—a common chemo drug—it starts co-assembling into droplets via liquid-liquid phase separation. Some mutants don’t even need the drug to phase separate—they do it all on their own. [R273C] stays fluid; [R273L] goes solid. These phase transitions could be the missing link to understanding chemo resistance and cancer relapse.
From cancer to condensation, our work lives at the edge of protein chaos. [Colloids and Surfaces B: Biointerfaces 212, 112371; Biophysical journal 118 (3), 720-728]
-
Packing Proteins with Purpose: The Microcompartment Chronicles
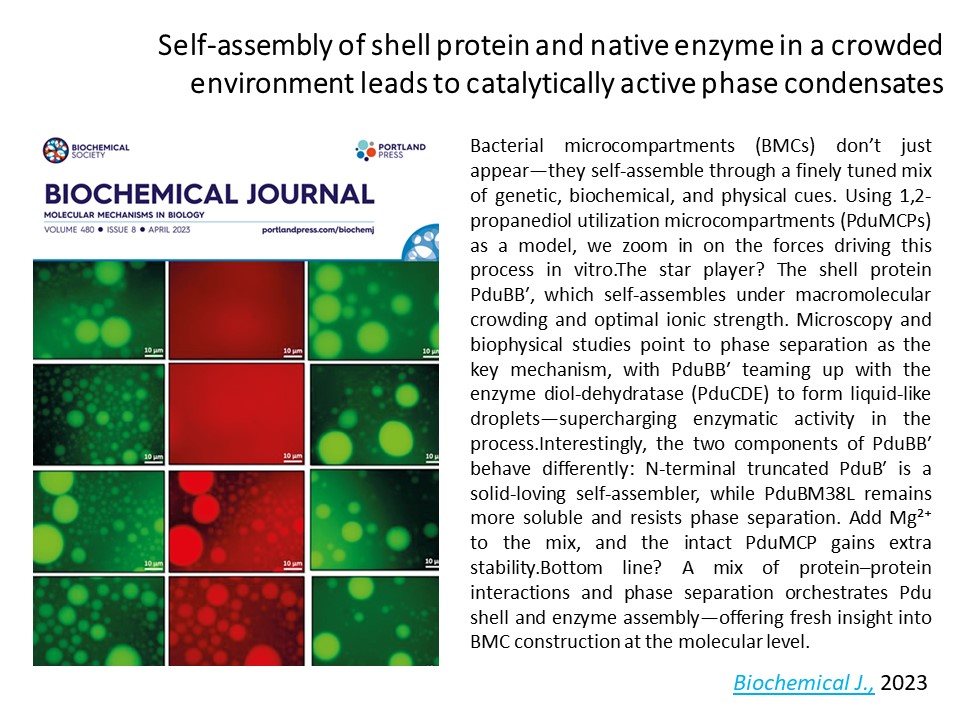
At the Sinha Lab, we decode how bacteria build their protein-based nanofactories—bacterial microcompartments (MCPs). Studying the pdu operon, we found that gene order isn’t about sequence similarity but how strongly the proteins interact—genome layout tuned for efficient assembly. We showed that Pdu shell proteins self-assemble via phase separation, forming enzyme-loaded droplets that boost catalytic activity. These shell proteins also moonlight as bioelectronic materials, generating photocurrent under UV light and revealing a proton-coupled electron transfer mechanism enhanced by genetic tweaks. From operon logic to light-harvesting protein pods, we’re revealing how nature builds and powers its own molecular machines. [BBA-General Subjects 1869 (6), 130794; bioRxiv, 2024.12. 16.628711; Journal of Materials Chemistry. B, 4842-4854; Biochemical Journal 480 (8), 539-553]
-
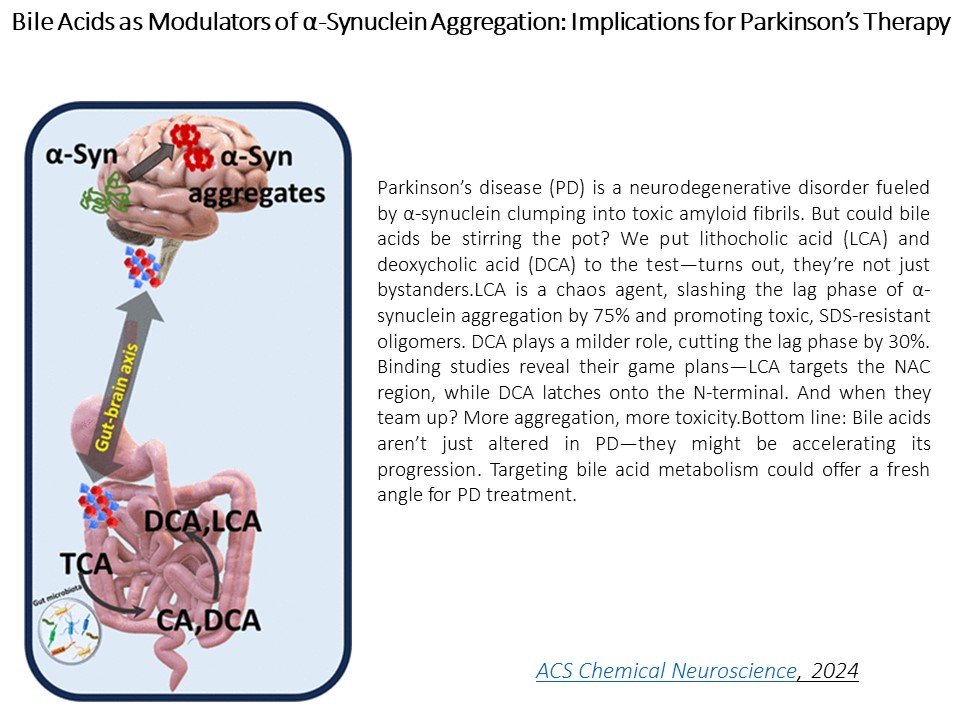
Role of Bile Acids and Doxorubicin in Parkinson's Disease
At the Sinha Lab, we’re on a mission to crack the molecular mysteries behind Parkinson’s disease (PD)—and our suspect list goes beyond the brain. It turns out the gut and cancer drugs might be stirring the pot. We've discovered that bile acids, like lithocholic acid (LCA) and deoxycholic acid (DCA)—usually busy helping digestion—are also busy hijacking α-synuclein. LCA binds to its sticky NAC region and slashes the aggregation lag time by 75%, forming SDS-resistant, toxic oligomers. DCA’s a bit gentler but still speeds things up. And when LCA and DCA join forces? It’s a molecular mess—more tangles, more toxicity. This gut-brain link might be a key player in PD pathogenesis.
But the surprises don’t stop there. Our lab also found that doxorubicin, a widely used chemotherapy drug, interacts with α-synuclein and destabilizes its structure, leading to aggregation—a possible explanation for Parkinson’s-like symptoms in some cancer patients. Interestingly, when L-DOPA, the gold-standard PD medication, enters the scene, it puts the brakes on aggregation. This points toward a new layer of neuroprotection—not just through dopamine, but by directly interfering with protein misfolding.
From bile acids to chemo cocktails, we're decoding how external triggers push α-synuclein over the edge—and how we might pull it back. If you’re curious about how gut chemistry, drugs, and rogue proteins collide, you’ll feel right at home here.[Current Research in Structural Biology 7, 100133; ACS Chemical Neuroscience 15 (21), 4055-4065]
-
Protein Bioelectronics
Our team is pioneering nanotechnology to tackle pressing biomedical challenges, with a recent breakthrough in early Parkinson’s Disease (PD) detection. We have developed a novel biosensor using specially engineered gold nanoclusters (AuNCs) that can selectively distinguish between healthy and toxic forms of the α-synuclein protein, a key PD biomarker. This sensitive platform can identify pathological changes before clinical symptoms appear, paving the way for early intervention. Our work, conducted with collaborators at CSIR-IMTECH and published in Nanoscale, has been featured in a press release from the Department of Science and Technology (DST), and covered by The Tribune India and Daily India. These highlights underscore our commitment to creating innovative tools that can revolutionize diagnostics and improve patient outcomes for neurodegenerative diseases.
https://ddindia.co.in/2025/08/tiny-gold-nanoparticles-show-promise-in-early-detection-of-parkinsons-disease/
https://www.tribuneindia.com/news/chandigarh/mohali-chandigarh-scientists-uncover-gold-nanoparticles-potential-in-early-detection-of-parkinsons/
https://dst.gov.in/tiny-gold-particles-can-help-early-detection-parkinsons-disease -
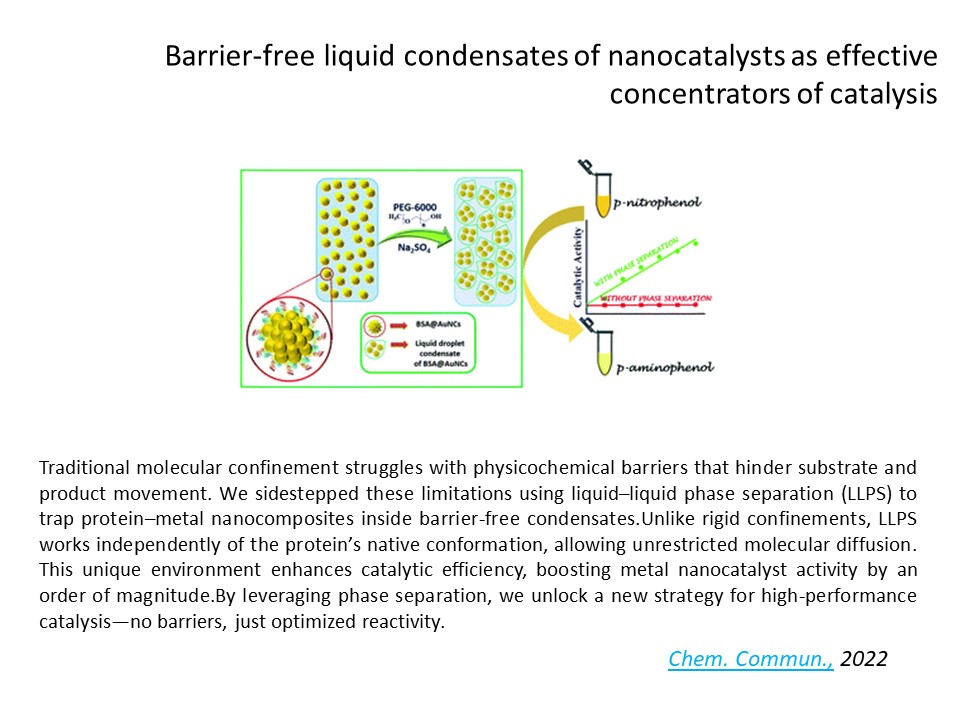
Catalysis in a Droplet
https://dst.gov.in/catalytic-droplets-leading-faster-chemical-reactions-can-bring-quicker-access-innovative-medications
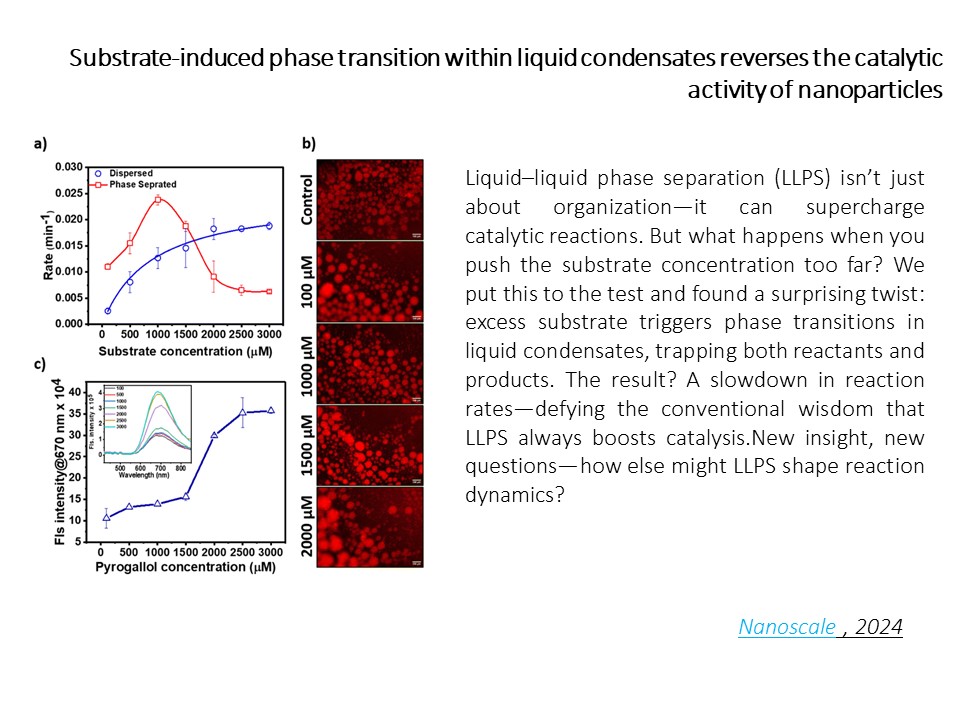
https://dst.gov.in/catalytic-droplets-leading-faster-chemical-reactions-can-bring-quicker-access-innovative-medicationsAt the Sinha Lab, we’re giving catalysis a new home: inside liquid droplets. No walls, no barriers—just phase-separated goo that turbocharges chemical reactions. Our experiments show that liquid–liquid phase separation (LLPS) can boost metal nanocatalyst activity by nearly 10x, all without needing fancy protein folding or rigid enclosures. Think of it as nature’s soft reactor—wet, wiggly, and wildly efficient.
But it’s not all fast lanes. We found that when the substrate concentration gets too high, the droplet interior shifts—slowing things down instead of speeding them up. These substrate-induced phase transitions jam molecular traffic inside the droplet, reducing the movement of both reactants and products—and surprisingly, this bottleneck lowers the reaction rate. It’s a twist no one saw coming.
So whether droplets act like boosters or bottlenecks depends on just the right molecular crowd. We're now decoding how squishy confinement rewires chemistry—opening up new routes in nanocatalysis, synthetic biology, and even smart drug delivery. [Chem. Commun., 2022,58, 8634-8637; Nanoscale 16 (31), 14730-14733]
Current Group Members
-

KAVITA VERMA
Email: kavita.ph25227@inst.ac.in
Reg. No.: PH25227
-

HIMANSHU RAJPUT
Email: himanshu.ar250101@inst.ac.in
Reg. No.: 10BB25J67001
-

ISHANI SHARMA
Email: ishani.ph23228@inst.ac.in
Reg. No.: PH23228
-

MR. S M ROSE
Email: smrose.ph21234@inst.ac.in
Reg. No.: PH21234
-

MS. AARCHA RADHAKRISHNAN
Email: aarcha.ph21202@inst.ac.in
Reg. No.: PH21202
-

MS. PREETI NEGI
Email: preeti.ph20209@inst.ac.in
Reg. No.: PH20209
-

MS. DIMPLE GOEL
Email: dimple.ph20210@inst.ac.in
Reg. No.: PH20210
Alumni
-

BHAVIKA ARORA
Reg. No.: IN-2024/05
Designation: Intern Student
Jan 2024 - Jun 2024
-

MS.SIMERPREET KAUR
Reg. No.: PH17224
Designation: PhD Scholar
Jan 2018 - Mar 2024
-

MS. HARPREET KAUR
Reg. No.: PH19201
Designation: PhD Scholar
Aug 2019 - Apr 2025
-

MS. SILKY
Reg. No.: PH19202
Designation: PhD Scholar
Aug 2019 - Apr 2025
-

MR. GAURAV KUMAR
Reg. No.: Ph17202
Designation: PhD Scholar
Aug 2017 - Feb 2023
-

MS. ANKUSH GARG
Reg. No.: PH15206
Designation: PhD Scholar
Aug 2015 - Dec 2021
-

MR. NAIMAT KALIM BARI
Reg. No.: PH14210
Designation: PhD Scholar
Aug 2014 - Jul 2020
-
1.
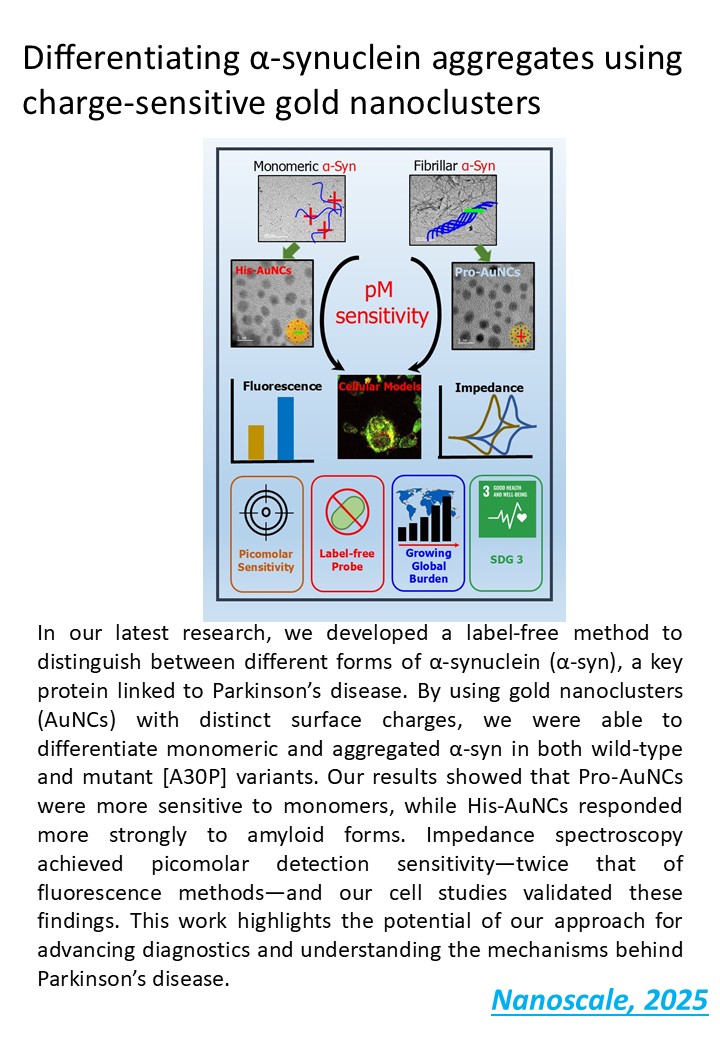
Differentiating α-synuclein aggregates using charge-sensitive gold nanoclusters , Harpreet Kaur, Arpit Tyagi, Ishani Sharma, Deepak Sharma and Sharmistha Sinha , Nanoscale , just accepted , just accepted , just accepted , https://doi.org/10.1039/D5NR00887E -
2.
Protein-protein interactions as determinants of operon architecture , Silky Bedi , S.M. Rose , Simerpreet Kaur 1 , Preeti Negi 1 , Sharmistha Sinha , BBA Gen Subjects , 2025 , 6 , 130794 , https://doi.org/10.1016/j.bbagen.2025.130794 -
3.
Bile Acids as Modulators of α-Synuclein Aggregation: Implications for Parkinson’s Therapy , H Kaur, D Swadia, S Sinha , ACS Chemical Neuroscience , 2024 , 15 (21), , 4055-4065 , https://doi.org/10.1021/acschemneuro.4c00459 -
4.
Substrate-induced phase transition within liquid condensates reverses the catalytic activity of nanoparticles , SM Rose, S Bedi, S Rakshit, S Sinha , Nanoscale , 2024 , 16(31) , 14730-14733 , https://doi.org/10.1039/D4NR01402B -
5.
Doxorubicin catalyses self-assembly of p53 by phase separation , A Garg, G Kumar, V Singh, S Sinha , Current Research in Structural Biology , 2023 , 7 , 100133 , https://doi.org/10.1016/j.crstbi.2024.100133 -
6.
Innate and engineered attributes of bacterial microcompartments for applications in bio-materials science , SM Rose, A Radhakrishnan, S Sinha , Journal of Materials Chemistry. B , 2023 , - , 4842-4854 , https://doi.org/10.1039/D3TB00098B -
7.
Self-assembly of Shell Protein and Native Enzyme in a Crowded Environment Leads to Catalytically Active Phase Condensates: , Gaurav Kumar, Sharmistha Sinha , (2023) , just accepted: just accepted , Biochemical Journal , 10.1042/BCJ20220551 -
8.
Disordered regions endow structural flexibility to shell proteins and function towards shell-enzyme interactions in propane diol utilization microcompartment: , Gaurav kumar, Jagadish Prasad Hazra, Sharmistha Sinha , (2022) , just accepted: , Journal of Biomolecular Structure & Dynamics -
9.
Barrier-free liquid condensates of nanocatalysts as effective concentrators of catalysis: , Silky Bedi, Gaurav Kumar, S. M. Rose, Sabyasachi Rakshit, and Sharmistha Sinha , (2022) , 58: 8634-8637 , Chemical Communications , DOI https://doi.org/10.1039/D2CC03111F -
10.
A Major Shell Protein of 1, 2‐Propanediol Utilization Microcompartment Conserves the Activity of Its Signature Enzyme at Higher Temperatures: , Gaurav Kumar, Naimat K. Bari,Jagadish P. Hazra,Dr. Sharmistha Sinha , (2022) , 23,: e2021006. , ChemBioChem , https://doi.org/10.1002/cbic.202100694 -
11.
Varying protein architectures in 3-dimensions for scaffolding and modulating properties of catalytic gold nanoparticles: , Simerpreet Kaur,Naimat K Bari,Sharmistha Sinha , (2022) , Just accepted: Just accepted , Amino Acids , 10.1007/s00726-022-03127-7 -
12.
Doxorubicin induced aggregation of α-synuclein: Insights into the mechanism of drug induced Parkinsonism: , Ankush Garg,Sharmistha Sinha , (2022) , 212: 112371. , Colloids and Surfaces B: Biointerfaces , 10.1016/j.colsurfb.2022.112371 -
13.
Biophysical approaches to understand and re-purpose bacterial microcompartments: , Gaurav Kumar,Sharmistha Sinha , (2021) , 63,: 43-51. , Current Opinion in Microbiology , 10.1016/j.mib.2021.05.008 -
14.
Factors deciding the assembly and thermostability of the DmrB cage: , Ankush Garg,Sharmistha Sinha , (2021) , 959-967, 182: 959-967 , International Journal of Biological Macromolecules 182 -
15.
Naturally occurring protein nano compartments: basic structure, function, and genetic engineering: , Dimple Goel,Sharmistha Sinha , (2021) , 2 (4): 042001 , Nano Express , 10.1088/2632-959X/ac2c93/ -
16.
Protein morphology drives the structure and catalytic activity of bio-inorganic hybrids: , Harpreet Kaur,Naimat K Bari,Ankush Garg,Sharmistha Sinha , (2021) , Just Accepted: , International Journal of Biological Macromolecules , 10.1016/j.ijbiomac.2021.01.217 -
17.
Temporal control in tritylation reactions through light-driven variation in chloride ion binding catalysis–A proof of concept: , Surbhi Grewal Saonli Roy Himanshu Kumar Mayank Saraswat Naimat K Bari Sharmistha Sinha Sugumar Venkataramani , (2020) , 10 (20): 7027-7033. , Catalysis Science & Technology , 10.1039/D0CY01090A -
18.
Probe into a Multi-Protein Prokaryotic Organelle Using Thermal Scanning Assay Reveals Distinct Properties of the Core and the Shell: , Dr. Naimat K Bari ,Jagadish P Hazra ,Gaurav Kumar,Simerpreet Kaur,SHARMISTHA SINHA , (2020) , 1864(10): 129680 , BBA - General Subjects , 10.1016/j.bbagen.2020.129680 -
19.
Variable Mutations at the p53-R273 Oncogenic Hotspot Position Leads to Altered Properties: , Ankush Garg , Jagadish Prasad Hazra 2, Malay Kumar Sannigrahi 2, Sabyasachi Rakshit 3, Sharmistha Sinha , (2019) , 118 (3): 720-728 , Biophysical Journal , 10.1016/j.bpj.2019.12.015 -
20.
Functional protein shells fabricated from the self-assembling protein sheets of prokaryotic organelles: , Naimat K. Bari, Gaurav Kumar , Jagadish P. Hazra, Simerpreet Kaur ,Sharmistha Sinha , (2019) , 8: 523-533 , Journal of Materials Chemistry B , https://doi.org/10.1039/C9TB02224D -
21.
Cellulose-metallothionein matrix for metal binding: , Naimat K.Bari ,Shaswat Barua ,Ankush Garg,Malay K Sannigrahi,Sharmistha Sinha , (2018) , 192: 126-134 , Carbohydrate polymers , 10.1016/j.carbpol.2018.03.043 -
22.
Nanoparticle Fabrication on Bacterial Microcompartment Surface for the Development of Hybrid Enzyme-Inorganic Catalyst.: , Naimat Kalim Bari , Gaurav Kumar, Aashish Bhatt, Jagadish Prasad Hazra, Ankush Garg, Md. Ehesan Ali , (2018) , 8: 7742–7748 , ACS Catal. , 10.1021/acscatal.8b02322 -
23.
The Wrappers of the 1,2-Propanediol Utilization Bacterial Microcompartments.: , Naimat K Bari, Gaurav Kumar, Sharmistha Sinha , (2018) , 1112: 333-344. , Adv Exp Med Biol , 10.1007/978-981-13-3065-0_23 -
24.
Enhanced bacterial cellulose production from Gluconobacter xylinus using super optimal broth: , Prathna T. Chandrasekaran, Naimat Kalim Bari , (2017) , 24(10): 4367–4381 , Cellulose -
25.
Selective molecular transport through the protein shell of a bacterial microcompartment organelle: , Chowdhury C, Chun S, Pang A, Sawaya MR, Sinha S, Yeates TO, Bobik TA , (2015) , 112(10): 2990-5. , Proc Natl Acad Sci U S A. , 10.1073/pnas.1423672112 -
26.
Alanine scanning mutagenesis identifies an asparagine-arginine-lysine triad essential to assembly of the shell of the Pdu microcompartment: , Shouqiang Cheng, Yea Won Sung, Dan E. McNamara, Michael R. Sawaya, Todd O. Yeates, Thomas A. Bobik. J. , (2014) , 26(12): 2328-45. , J. Mol. Biol. , 10.1016/j.jmb.2014.04.012 -
27.
Diverse bacterial microcompartment organelles: , Chowdhury C, Sinha S, Chun S, Yeates TO, Bobik TA , (2014) , 78(3): 438-68 , Microbiol Mol Biol Rev , 10.1128/MMBR.00009-14 -
28.
A Key Role for Lysine Residues in Amyloid β-Protein Folding, Assembly, and Toxicity: , Sharmistha Sinha,Dahabada H. J. Lopes,Gal Bitan. ACS Chem. Neurosci. , (2012) , 3 (6): 473–481. , ACS Chem. Neurosci. , 10.1021/cn3000247 -
29.
Interactions between the termini of lumen enzymes and shell proteins mediate enzyme encapsulation into bacterial microcompartments: , Sharmistha Sinha , (2012) , 109(37): 14995-5000. , PNAS , 10.1073/pnas.1207516109 -
30.
Comparison of Three Amyloid Assembly Inhibitors: The Sugar Scyllo-Inositol, the Polyphenol Epigallocatechin Gallate, and the Molecular Tweezer CLR01: , Sharmistha Sinha 1, Zhenming Du, Panchanan Maiti, Frank-Gerrit Klärner, Thomas Schrader, Chunyu Wang, Gal Bitan , (2012) , 3(6): 451-8. , ACS Chemical Neuroscience , DOI: 10.1021/cn200133x -
31.
Lysine-Specific Molecular Tweezers Are Broad-Spectrum Inhibitors of Assembly and Toxicity of Amyloid Proteins: , Sharmistha Sinha, Dahabada H. J. Lopes, Zhenming Du, Eric S. Pang, Akila Shanmugam, Aleksey Lomakin, Peter Talbiersky, Annette Tennstaedt, Kirsten McDaniel, Reena Bakshi, Pei-Yi Kuo, Michael Ehrmann, George B. Benedek, Joseph A. Loo, Frank-Gerrit Klärner, Thomas Schrader, Chunyu Wang, and Gal Bitan J. Am. Chem. Soc , (2011) , 133 (42): 16958–16969. , J. Am. Chem. Soc. , 10.1021/ja206279b
-
1.
Application of Photochemical Cross-linking to the Study of Oligomerization of Amyloidogenic Proteins: , Lopes DHJ, C Rosensweig, G Bitan , (2012) , 849: 11-21 , Methods Mol Biol. -
2.
Amyloids and Protein Aggregation—Analytical Methods: , Li H, F Rahimi, K Murakami, P Maiti, G Bitan , (2009) , Encyclopedia of Analytical Chemistry
-
1.
The 56th Biophysical Society Meeting: , SanDiego, CA , (2012) -
2.
The 23rd Symposium of the Protein Society, Boston: , MA , (2009) -
3.
XVII International Symposium on Glycoconjugates (GLYCO-17): , Bangalore , (2002)
- 1. Xviii International Symposium On Glycoconjugates (glyco-18): , Florence, Italy, (2005)
- 2. Molecular Tweezers For The Treatment Of Amyloid-related Diseases: , P. Talbiersky, A. Lomakin, F.-G. Klärner, T. Schrader, S. Frautschy,G. Bitan , (2010)
- 3. New Compounds For The Treatment Of Diseases Related To Protein Misfolding: , T Schrader, K Hochdorffer, J Marz-Berberich, L Nagel-Steger, G Bitan , (2010)
Research Associate: Indian Institute of Science, Bangalore,, India (April 2007 to April 2008 )
Postdoctoral Fellow: Dept. of Neurology, University of California, Los Angeles,, USA (March 2008 to March 2010 )
Postdoctoral Fellow: Dept. of Biochem. Biophys. and Mol. Biol., Iowa State University, Ames,, USA (April 2010 to April 2013)
Recipient of the Har Govind Khorana-Innovative Young Biotechnologist Award , 2019, Department of Biotechnology, Govt. of India http://dbtindia.gov.in/sites/default/files/List_final_selected_HGK-IYBA_2019_Awardees_0_0.pdf
Recipient of the SERB Women Excellence Award, 2017, Department of Science and Technology, Government of India
Recipient of the INSA Medal for Young Scientist 2008, Indian National Science Academy, Government of India.
-
Title: Cell-free bioreactors from the shell proteins of bacterial microcompartments
PI: Prof. Sharmistha Sinha
Funding Amount: 47 lakhs
Tenure: 3 years
Funding Agency: SERB
-
Title: Cellulose-protein binary conjugates for metal detoxification
PI: Prof. Sharmistha Sinha
Funding Amount: 18 lakhs
Tenure: 3 years
Funding Agency: SERB-Women Excellence Award
-
Title: Exploring the shell proteins of BMCPs as potential substrate for fabrication of Organic-Inorganic Hybrid Nanomaterials
PI: Prof. Sharmistha Sinha
CO-PI: Prof. Ehesan Ali
Funding Amount: 47 lakhs
Tenure: 3 years
Funding Agency: DST Nano Mission
-
Title: Understanding the Forces Involved in the Packing of Enzymes Inside the Bacterial Microcompartments for the Development of Novel Encapsulated Bio-Systems
PI: Prof. Sharmistha Sinha
Funding Amount: 58 lakhs
Tenure: 3 years
Funding Agency: DBT
-
Title: Biological treatment of engineered nanomaterials-contaminated wastewater-feasibility and implication
PI: Dr. Sonalika Vaidya Role
CO-PI: Prof. Sharmistha Sinha
Funding Amount: 10 Lakhs (completed)
Tenure: 2 years
Funding Agency: INST